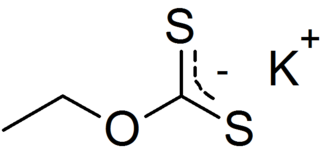In organic chemistry, a thiol, or thiol derivative, is any organosulfur compound of the form R−SH, where R represents an alkyl or other organic substituent. The −SH functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols, and the word is a blend of "thio-" with "alcohol".
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to chemical compounds large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.

Ethylene oxide is an organic compound with the formula C2H4O. It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of silver catalyst.

Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and MnO−
4, an intensely pink to purple solution.

Ethanethiol, commonly known as ethyl mercaptan, is an organosulfur compound with the formula CH3CH2SH. is a colorless liquid with a distinct odor. Abbreviated EtSH, it consists of an ethyl group (Et), CH3CH2, attached to a thiol group, SH. Its structure parallels that of ethanol, but with sulfur in place of oxygen. The odor of EtSH is infamous. Ethanethiol is more volatile than ethanol due to a diminished ability to engage in hydrogen bonding. Ethanethiol is toxic in high concentrations. It occurs naturally as a minor component of petroleum, and may be added to otherwise odorless gaseous products such as liquefied petroleum gas (LPG) to help warn of gas leaks. At these concentrations, ethanethiol is not harmful.

Bisulfide is an inorganic anion with the chemical formula HS−. It contributes no color to bisulfide salts, and its salts may have a distinctive putrid smell. It is a strong base. Bisulfide solutions are corrosive and attack the skin.

Sodium azide is an inorganic compound with the formula NaN3. This colorless salt is the gas-forming component in some car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance, is highly soluble in water, and is very acutely poisonous.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.

Tollens' reagent is a chemical reagent used to distinguish between aldehydes and ketones along with some alpha-hydroxy ketones which can tautomerize into aldehydes. The reagent consists of a solution of silver nitrate, ammonium hydroxide and some sodium hydroxide. It was named after its discoverer, the German chemist Bernhard Tollens. A positive test with Tollens' reagent is indicated by the precipitation of elemental silver, often producing a characteristic "silver mirror" on the inner surface of the reaction vessel.

Froth flotation is a process for selectively separating hydrophobic materials from hydrophilic. This is used in mineral processing, paper recycling and waste-water treatment industries. Historically this was first used in the mining industry, where it was one of the great enabling technologies of the 20th century. It has been described as "the single most important operation used for the recovery and upgrading of sulfide ores". The development of froth flotation has improved the recovery of valuable minerals, such as copper- and lead-bearing minerals. Along with mechanized mining, it has allowed the economic recovery of valuable metals from much lower grade ore than previously.

Xanthate usually refers to a salt of xanthic acid. The formula of the salt of xanthic acid is [R−O−CS2]−M+ ,. Xanthate also refers to the anion [R−O−CS2]−. Xanthate also may refer to an ester of xanthic acid. The formula of xanthic acid is R−O−C(=S)−S−H, while the formula of the esters of xanthic acid is R−O−C(=S)−S−R', where R and R' are organyl groups. The salts of xanthates are also called O-organyl dithioates. The esters of xanthic acid are also called O,S-diorganyl esters of dithiocarbonic acid. The name xanthate is derived from Ancient Greek ξανθός xanthos, meaning “yellowish, golden”, and indeed most xanthate salts are yellow. They were discovered and named in 1823 by Danish chemist William Christopher Zeise. These organosulfur compounds are important in two areas: the production of cellophane and related polymers from cellulose and for extraction of certain sulphide bearing ores. They are also versatile intermediates in organic synthesis.

Thioglycolic acid (TGA) is the organic compound HSCH2CO2H. TGA is often called mercaptoacetic acid (MAA). It contains both a thiol (mercaptan) and carboxylic acid functional groups. It is a colorless liquid with a strongly unpleasant odor. TGA is miscible with polar organic solvents.
A Nitrate Test is a chemical test used to determine the presence of nitrate ion in solution. Testing for the presence of nitrate via wet chemistry is generally difficult compared with testing for other anions, as almost all nitrates are soluble in water. In contrast, many common ions give insoluble salts, e.g. halides precipitate with silver, and sulfate precipitate with barium.

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements.
Thiocarbonate describes a family of anions with the general chemical formula CS
3−xO2−
x (x = 0, 1, or 2):
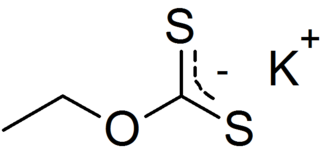
Potassium ethyl xanthate (KEX) is an organosulfur compound with the chemical formula CH3CH2OCS2K. It is a pale yellow powder that is used in the mining industry for the separation of ores. It is a potassium salt of ethyl xanthic acid.
Silver hyponitrite is an ionic compound with formula Ag2N2O2 or (Ag+
)2[ON=NO]2−, containing monovalent silver cations and hyponitrite anions. It is a bright canary yellow solid practically insoluble in water and most organic solvents, including DMF and DMSO.
Potassium amyl xanthate (/pəˈtæsiəm ˌæmɪl ˈzænθeɪt/) is an organosulfur compound with the chemical formula CH3(CH2)4OCS2K. It is a pale yellow powder with a pungent odor that is soluble in water. It is widely used in the mining industry for the separation of ores using the flotation process.

Diethyl dixanthogen disulfide is the organosulfur compound with the formula (C2H5OC S)2. It is one of the most common dixanthogen disulfides, compounds of the type (ROC S)2. A yellow solid, It is obtained by oxidation of sodium ethylxanthate or potassium ethylxanthate.