In organic chemistry, the Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide (DMSO) and an organic base, such as triethylamine. It is one of the many oxidation reactions commonly referred to as 'activated DMSO' oxidations. The reaction is known for its mild character and wide tolerance of functional groups.
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2-dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates.

Sodium hydride is the chemical compound with the empirical formula NaH. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in contrast to molecular hydrides such as borane, methane, ammonia, water, and hydrogen fluoride. It is an ionic material that is insoluble in all solvents (other than molten Na), consistent with the fact that H− ions do not exist in solution. Because of the insolubility of NaH, all reactions involving NaH occur at the surface of the solid.
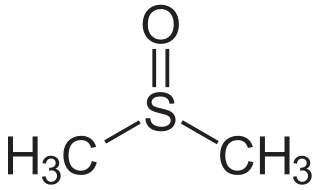
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula (CH3)2SO. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high boiling point. DMSO has the unusual property that many individuals perceive a garlic-like taste in the mouth after DMSO makes contact with their skin.

Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula NaBH4. It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodium borohydride is a reducing agent that finds application in papermaking and dye industries. It is also used as a reagent in organic synthesis.
The Robinson annulation is a chemical reaction used in organic chemistry for ring formation. It was discovered by Robert Robinson in 1935 as a method to create a six membered ring by forming three new carbon–carbon bonds. The method uses a ketone and a methyl vinyl ketone to form an α,β-unsaturated ketone in a cyclohexane ring by a Michael addition followed by an aldol condensation. This procedure is one of the key methods to form fused ring systems.
The Simmons–Smith reaction is an organic cheletropic reaction involving an organozinc carbenoid that reacts with an alkene to form a cyclopropane. It is named after Howard Ensign Simmons, Jr. and Ronald D. Smith. It uses a methylene free radical intermediate that is delivered to both carbons of the alkene simultaneously, therefore the configuration of the double bond is preserved in the product and the reaction is stereospecific.

In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent.

The Johnson–Corey–Chaykovsky reaction is a chemical reaction used in organic chemistry for the synthesis of epoxides, aziridines, and cyclopropanes. It was discovered in 1961 by A. William Johnson and developed significantly by E. J. Corey and Michael Chaykovsky. The reaction involves addition of a sulfur ylide to a ketone, aldehyde, imine, or enone to produce the corresponding 3-membered ring. The reaction is diastereoselective favoring trans substitution in the product regardless of the initial stereochemistry. The synthesis of epoxides via this method serves as an important retrosynthetic alternative to the traditional epoxidation reactions of olefins.
The Julia olefination (also known as the Julia–Lythgoe olefination) is the chemical reaction used in organic chemistry of phenyl sulfones (1) with aldehydes (or ketones) to give alkenes (olefins)(3) after alcohol functionalization and reductive elimination using sodium amalgam or SmI2. The reaction is named after the French chemist Marc Julia.

Diethylaminosulfur trifluoride (DAST) is the organosulfur compound with the formula Et2NSF3. This liquid is a fluorinating reagent used for the synthesis of organofluorine compounds. The compound is colourless; older samples assume an orange colour.
Luche reduction is the selective organic reduction of α,β-unsaturated ketones to allylic alcohols. The active reductant is described as "cerium borohydride", which is generated in situ from NaBH4 and CeCl3(H2O)7.
The Parikh–Doering oxidation is an oxidation reaction that transforms primary and secondary alcohols into aldehydes and ketones, respectively. The procedure uses dimethyl sulfoxide (DMSO) as the oxidant and the solvent, activated by the sulfur trioxide pyridine complex (SO3•C5H5N) in the presence of triethylamine or diisopropylethylamine as base. Dichloromethane is frequently used as a cosolvent for the reaction.
Alcohol oxidation is a collection of oxidation reactions in organic chemistry that convert alcohols to aldehydes, ketones, carboxylic acids, and esters where the carbon carries a higher oxidation state. The reaction mainly applies to primary and secondary alcohols. Secondary alcohols form ketones, while primary alcohols form aldehydes or carboxylic acids.
Desulfonylation reactions are chemical reactions leading to the removal of a sulfonyl group from organic compounds. As the sulfonyl functional group is electron-withdrawing, methods for cleaving the sulfur–carbon bonds of sulfones are typically reductive in nature. Olefination or replacement with hydrogen may be accomplished using reductive desulfonylation methods.

Trimethylsulfoxonium iodide is a sulfoxonium salt. It is used to generate dimethyloxosulfonium methylide by reaction with sodium hydride. The latter compound is used as a methylene-transfer reagent, and is used to prepare epoxides.
The Pinnick oxidation is an organic reaction by which aldehydes can be oxidized into their corresponding carboxylic acids using sodium chlorite (NaClO2) under mild acidic conditions. It was originally developed by Lindgren and Nilsson. The typical reaction conditions used today were developed by G. A. Kraus. H.W. Pinnick later demonstrated that these conditions could be applied to oxidize α,β-unsaturated aldehydes. There exist many different reactions to oxidize aldehydes, but only a few are amenable to a broad range of functional groups. The Pinnick oxidation has proven to be both tolerant of sensitive functionalities and capable of reacting with sterically hindered groups. This reaction is especially useful for oxidizing α,β-unsaturated aldehydes, and another one of its advantages is its relatively low cost.
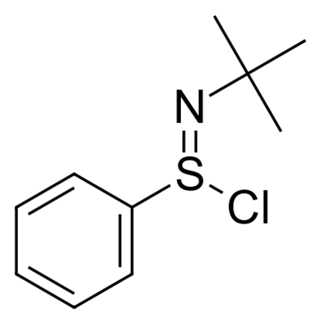
N-tert-Butylbenzenesulfinimidoyl chloride is a useful oxidant for organic synthesis reactions. It is a good electrophile, and the sulfimide S=N bond can be attacked by nucleophiles, such as alkoxides, enolates, and amide ions. The nitrogen atom in the resulting intermediate is basic, and can abstract an α-hydrogen to create a new double bond.

Trifluoroperacetic acid is an organofluorine compound, the peroxy acid analog of trifluoroacetic acid, with the condensed structural formula CF
3COOOH. It is a strong oxidizing agent for organic oxidation reactions, such as in Baeyer–Villiger oxidations of ketones. It is the most reactive of the organic peroxy acids, allowing it to successfully oxidise relatively unreactive alkenes to epoxides where other peroxy acids are ineffective. It can also oxidise the chalcogens in some functional groups, such as by transforming selenoethers to selones. It is a potentially explosive material and is not commercially available, but it can be quickly prepared as needed. Its use as a laboratory reagent was pioneered and developed by William D. Emmons.
Sulfonium-based oxidations of alcohols to aldehydes summarizes a group of organic reactions that transform a primary alcohol to the corresponding aldehyde (and a secondary alcohol to the corresponding ketone). Selective oxidation of alcohols to aldehydes requires circumventing over-oxidation to the carboxylic acid. One popular approach are methods that proceed through intermediate alkoxysulfonium species (RO−SMe+
2X-, e.g. compound 6) as detailed here. Since most of these methods employ dimethylsulfoxide (DMSO) as oxidant and generate dimethylsulfide, these are often colloquially summarized as DMSO-oxidations. Conceptually, generating an aldehyde and dimethylsulfide from an alcohol and DMSO requires a dehydrating agent for removal of H2O, ideally an electrophile simultaneously activating DMSO. In contrast, methods generating the sulfonium intermediate from dimethylsulfide do not require a dehydrating agent. Closely related are oxidations mediated by dimethyl selenoxide and by dimethyl selenide.