Sodium laureth sulfate (SLES), an accepted contraction of sodium lauryl ether sulfate (SLES), also called sodium alkylethersulfate, is an anionic detergent and surfactant found in many personal care products and for industrial uses. SLES is an inexpensive and very effective foaming agent. SLES, sodium lauryl sulfate (SLS), ammonium lauryl sulfate (ALS), and sodium pareth sulfate are surfactants that are used in many cosmetic products for their cleaning and emulsifying properties. It is derived from palm kernel oil or coconut oil. In herbicides, it is used as a surfactant to improve absorption of the herbicidal chemicals and reduces time the product takes to be rainfast, when enough of the herbicidal agent will be absorbed.
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula CH3(CH2)11OSO3Na and structure H3C−(CH2)11−O−S(=O)2−O−Na+. It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium salt of the 12-carbon organosulfate. Its hydrocarbon tail combined with a polar "headgroup" give the compound amphiphilic properties that make it useful as a detergent. SDS is also component of mixtures produced from inexpensive coconut and palm oils. SDS is a common component of many domestic cleaning, personal hygiene and cosmetic, pharmaceutical, and food products, as well as of industrial and commercial cleaning and product formulations.

The sulfate or sulphate ion is a polyatomic anion with the empirical formula SO2−4. Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid.

Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word "surfactant" is a blend of surface-active agent, coined c. 1950. As they consist of a water-repellent and a water-attracting part, they enable water and oil to mix; they can form foam and facilitate the detachment of dirt.

Kala namak or black salt is a kiln-fired rock salt with a sulphurous, pungent smell used in the Indian subcontinent. It is also known as "Himalayan black salt", Sulemani namak, bit noon, bire noon, bit loona, bit lobon, kala loon, sanchal, kala meeth, guma loon, or pada loon, and is manufactured from the salts mined in the regions surrounding the Himalayas.

Magnesium sulfate or magnesium sulphate is a chemical compound, a salt with the formula MgSO4, consisting of magnesium cations Mg2+ (20.19% by mass) and sulfate anions SO2−4. It is a white crystalline solid, soluble in water but not in ethanol.

Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. It is mainly used as a filler in the manufacture of powdered home laundry detergents and in the Kraft process of paper pulping for making highly alkaline sulfides.
Ammonium lauryl sulfate (ALS) is the common name for ammonium dodecyl sulfate (CH3(CH2)10CH2OSO3NH4). The anion consists of a nonpolar hydrocarbon chain and a polar sulfate end group. The combination of nonpolar and polar groups confers surfactant properties to the anion: it facilitates dissolution of both polar and non-polar materials. This salt is classified as a sulfate ester. It is made from coconut or palm kernel oil for use primarily in shampoos and body-wash as a foaming agent. Lauryl sulfates are very high-foam surfactants that disrupt the surface tension of water in part by forming micelles at the surface-air interface.

Potassium sulfate (US) or potassium sulphate (UK), also called sulphate of potash (SOP), arcanite, or archaically potash of sulfur, is the inorganic compound with formula K2SO4, a white water-soluble solid. It is commonly used in fertilizers, providing both potassium and sulfur.
SLES is an initialism for:
Dodecanol, or lauryl alcohol, is an organic compound produced industrially from palm kernel oil or coconut oil. It is a fatty alcohol. Sulfate esters of lauryl alcohol, especially sodium lauryl sulfate, are very widely used as surfactants. Sodium lauryl sulfate and the related dodecanol derivatives ammonium lauryl sulfate and sodium laureth sulfate are all used in shampoos. Dodecanol is tasteless, colorless, and has a floral odor.
A foaming agent is a material such as a surfactant or a blowing agent that facilitates the formation of foam. A surfactant, when present in small amounts, reduces surface tension of a liquid or increases its colloidal stability by inhibiting coalescence of bubbles. A blowing agent is a gas that forms the gaseous part of the foam.

Sodium bisulfate, also known as sodium hydrogen sulfate, is the sodium salt of the bisulfate anion, with the molecular formula NaHSO4. Sodium bisulfate is an acid salt formed by partial neutralization of sulfuric acid by an equivalent of sodium base, typically in the form of either sodium hydroxide (lye) or sodium chloride (table salt). It is a dry granular product that can be safely shipped and stored. The anhydrous form is hygroscopic. Solutions of sodium bisulfate are acidic, with a 1M solution having a pH of slightly below 1.

Sodium persulfate is the inorganic compound with the formula Na2S2O8. It is the sodium salt of peroxydisulfuric acid, H2S2O8, an oxidizing agent. It is a white solid that dissolves in water. It is almost non-hygroscopic and has good shelf-life.
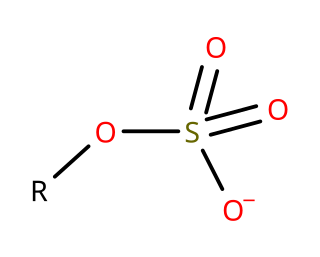
In organosulfur chemistry, organosulfates are a class of organic compounds sharing a common functional group with the structure R−O−SO−3. The SO4 core is a sulfate group and the R group is any organic residue. All organosulfates are formally esters derived from alcohols and sulfuric acid although many are not prepared in this way. Many sulfate esters are used in detergents, and some are useful reagents. Alkyl sulfates consist of a hydrophobic hydrocarbon chain, a polar sulfate group and either a cation or amine to neutralize the sulfate group. Examples include: sodium lauryl sulfate and related potassium and ammonium salts.

Acıgöl is a lake in Turkey's inner Aegean Region, in an endorheic basin at the junction between Denizli Province, Afyonkarahisar Province, and Burdur Province. Its surface area varies greatly through the seasons, with 100 km² in spring and 35 km² in late summer, with a maximum depth of 1.63 m. The lake is notable for its sodium sulfate reserves, extensively used in the industry, and Turkey's largest commercial sodium sulfate production operations are based here. It is situated 60 km east of Denizli city. From west to east, the lake's surrounding districts and towns are Bozkurt, Çardak, Dazkırı and Başmakçı.

Sodium aluminium sulfate is the inorganic compound with the chemical formula NaAl(SO4)2·12H2O (sometimes written Na2SO4·Al2(SO4)3·24H2O). Also known as soda alum, sodium alum, or SAS, this white solid is used in the manufacture of baking powder and as a food additive. Its official mineral name is alum-Na (IMA symbol: Aum-Na).
Sodium pyrosulfate is an inorganic compound with the chemical formula of Na2S2O7. It is a colorless salt. It hydrolyses in water to form sodium bisulfate with a chemical formula of NaHSO4 which has a pH of around 1.

Prasterone sulfate, also known as dehydroepiandrosterone sulfate (DHEA-S), is a naturally occurring androstane steroid which is marketed and used in Japan and other countries as a labor inducer in the treatment of insufficient cervical ripening and dilation during childbirth. It is the C3β sulfate ester of prasterone, and is known to act as a prohormone of DHEA and by extension of androgens and estrogens, although it also has its own activity as a neurosteroid. Prasterone sulfate is used medically as the sodium salt via injection and is referred to by the name sodium prasterone sulfate.











