
Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.

Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents.

Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.

Sodium hydride is the chemical compound with the empirical formula NaH. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in contrast to molecular hydrides such as borane, methane, ammonia, water, and hydrogen fluoride. It is an ionic material that is insoluble in all solvents (other than molten Na), consistent with the fact that H− ions do not exist in solution.

Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula NaBH4. It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodium borohydride is a reducing agent that finds application in papermaking and dye industries. It is also used as a reagent in organic synthesis.

Sodium bis(2-methoxyethoxy)aluminium hydride (SMEAH; trade names Red-Al, Synhydrid, Vitride) is a complex hydride reductant with the formula NaAlH2(OCH2CH2OCH3)2. The trade name Red-Al refers to its being a reducing aluminium compound. It is used predominantly as a reducing agent in organic synthesis. The compound features a tetrahedral aluminium center attached to two hydride and two alkoxide groups, the latter derived from 2-methoxyethanol. Commercial solutions are colorless/pale yellow and viscous. At low temperatures (below -60 °C), the solution solidifies to a glassy pulverizable substance with no sharp melting point.
Triethylborane (TEB), also called triethylboron, is an organoborane. It is a colorless pyrophoric liquid. Its chemical formula is (CH3CH2)3B or (C2H5)3B, abbreviated Et3B. It is soluble in organic solvents tetrahydrofuran and hexane.

Borohydride refers to the anion [BH4]−, which is also called tetrahydridoborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing [BH4−nXn]−, where n is an integer from 0 to 3, for example cyanoborohydride or cyanotrihydroborate [BH3(CN)]− and triethylborohydride or triethylhydroborate [BH(CH2CH3)3]−. Borohydrides find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known. Tetrahydroborates are also of academic and industrial interest in inorganic chemistry.
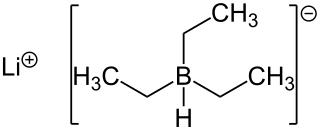
Lithium triethylborohydride is the organoboron compound with the formula LiEt3BH. Commonly referred to as LiTEBH or Superhydride, it is a powerful reducing agent used in organometallic and organic chemistry. It is a colorless or white liquid but is typically marketed and used as a THF solution. The related reducing agent sodium triethylborohydride is commercially available as toluene solutions.

Sodium aluminium hydride or sodium alumanuide is an inorganic compound with the chemical formula NaAlH4. It is a white pyrophoric solid that dissolves in tetrahydrofuran (THF), but not in diethyl ether or hydrocarbons. It has been evaluated as an agent for the reversible storage of hydrogen and it is used as a reagent for the chemical synthesis of organic compounds. Similar to lithium aluminium hydride, it is a salt consisting of separated sodium cations and tetrahedral AlH−
4 anions.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.

Diethylaluminium chloride, abbreviated DEAC, is an organoaluminium compound. Although often given the chemical formula (C2H5)2AlCl, it exists as a dimer, [(C2H5)2AlCl]2 It is a precursor to Ziegler-Natta catalysts employed for the production of polyolefins. The compound is also a Lewis acid, useful in organic synthesis. The compound is a colorless waxy solid, but is usually handled as a solution in hydrocarbon solvents. It is highly reactive, even pyrophoric.

Cobalt tetracarbonyl hydride is an organometallic compound with the formula HCo(CO)4. It is a volatile, yellow liquid that forms a colorless vapor and has an intolerable odor. The compound readily decomposes upon melt and in absentia of high CO partial pressures forms Co2(CO)8. Despite operational challenges associated with its handling, the compound has received considerable attention for its ability to function as a catalyst in hydroformylation. In this respect, HCo(CO)4 and related derivatives have received significant academic interest for their ability to mediate a variety of carbonylation (introduction of CO into inorganic compounds) reactions.
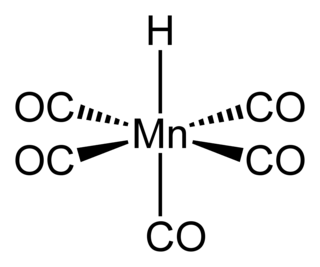
Pentacarbonylhydridomanganese is an organometallic compound with formula HMn(CO)5. This compound is one of the most stable "first-row" transition metal hydrides.

In organic chemistry, carbonyl reduction is the conversion of any carbonyl group, usually to an alcohol. It is a common transformation that is practiced in many ways. Ketones, aldehydes, carboxylic acids, esters, amides, and acid halides - some of the most pervasive functional groups, -comprise carbonyl compounds. Carboxylic acids, esters, and acid halides can be reduced to either aldehydes or a step further to primary alcohols, depending on the strength of the reducing agent. Aldehydes and ketones can be reduced respectively to primary and secondary alcohols. In deoxygenation, the alcohol group can be further reduced and removed altogether by replacement with H.

Ethylaluminium sesquichloride, also called EASC, is an industrially important organoaluminium compound used primarily as a precursor to triethylaluminium and as a catalyst component in Ziegler–Natta type systems for olefin and diene polymerizations. Other applications include use in alkylation reactions and as a catalyst component in linear oligomerization and cyclization of unsaturated hydrocarbons. EASC is a colourless liquid, spontaneously combustible in air and reacts violently when in contact with water and many other compounds.

Phenylsodium C6H5Na is an organosodium compound. Solid phenylsodium was first isolated by Nef in 1903. Although the behavior of phenylsodium and phenyl magnesium bromide are similar, the organosodium compound is very rarely used.

Octahydrotriborate is the boron hydride anion B3H8−. It forms a variety of salts that are colorless and air-stable. The tetrabutylammonium salt is soluble in organic solvents such as acetonitrile and methylene chloride. The anion is an intermediate is the synthesis of various higher boron hydrides, such as pentaborane(9). B3H8− can be viewed as the conjugate base of triborane B3H9.
Fluorohydride salts are ionic compounds containing a mixture of fluoride and hydride anions, generally with strongly electropositive metal cations. Unlike other types of mixed hydrides such as oxyhydrides, fluorohydride salts are typically solid solutions because of the similar sizes and identical charges of fluoride and hydride ions.
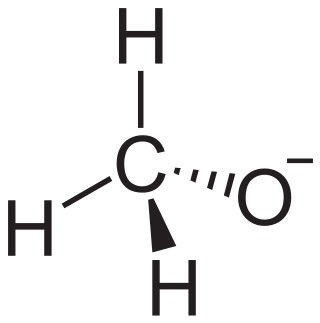
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as RO−, where R is the organyl substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis. Transition metal alkoxides are widely used for coatings and as catalysts.



















