Angiotensin-converting-enzyme inhibitors are a class of medication used primarily for the treatment of high blood pressure and heart failure. This class of medicine works by causing relaxation of blood vessels as well as a decrease in blood volume, which leads to lower blood pressure and decreased oxygen demand from the heart.
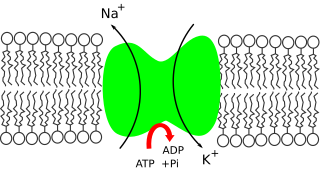
Potassium is the main intracellular ion for all types of cells, while having a major role in maintenance of fluid and electrolyte balance. Potassium is necessary for the function of all living cells, and is thus present in all plant and animal tissues. It is found in especially high concentrations within plant cells, and in a mixed diet, it is most highly concentrated in fruits. The high concentration of potassium in plants, associated with comparatively very low amounts of sodium there, historically resulted in potassium first being isolated from the ashes of plants (potash), which in turn gave the element its modern name. The high concentration of potassium in plants means that heavy crop production rapidly depletes soils of potassium, and agricultural fertilizers consume 93% of the potassium chemical production of the modern world economy.
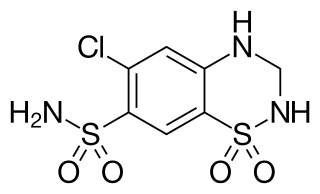
Hydrochlorothiazide, sold under the brand name Hydrodiuril among others, is a diuretic medication used to treat hypertension and swelling due to fluid build-up. Other uses include treating diabetes insipidus and renal tubular acidosis and to decrease the risk of kidney stones in those with a high calcium level in the urine. Hydrochlorothiazide is taken by mouth and may be combined with other blood pressure medications as a single pill to increase effectiveness. Hydrochlorothiazide is a thiazide medication which inhibits reabsorption of sodium and chloride ions from the distal convoluted tubules of the kidneys, causing a natriuresis. This initially increases urine volume and lowers blood volume. It is believed to reduce peripheral vascular resistance.

Quinidine is a class IA antiarrhythmic agent used to treat heart rhythm disturbances. It is a diastereomer of antimalarial agent quinine, originally derived from the bark of the cinchona tree. The drug causes increased action potential duration, as well as a prolonged QT interval. As of 2019, its IV formulation is no longer being manufactured for use in the United States.
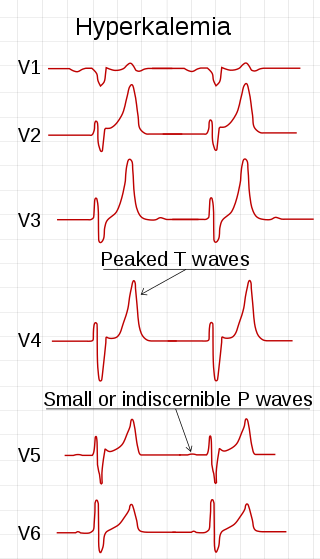
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0 mmol/L (3.5 and 5.0 mEq/L) with levels above 5.5 mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Occasionally when severe it can cause palpitations, muscle pain, muscle weakness, or numbness. Hyperkalemia can cause an abnormal heart rhythm which can result in cardiac arrest and death.

Electrolyte imbalance, or water-electrolyte imbalance, is an abnormality in the concentration of electrolytes in the body. Electrolytes play a vital role in maintaining homeostasis in the body. They help to regulate heart and neurological function, fluid balance, oxygen delivery, acid–base balance and much more. Electrolyte imbalances can develop by consuming too little or too much electrolyte as well as excreting too little or too much electrolyte. Examples of electrolytes include calcium, chloride, magnesium, phosphate, potassium, and sodium.
Hyperkalemic periodic paralysis is an inherited autosomal dominant disorder that affects sodium channels in muscle cells and the ability to regulate potassium levels in the blood. It is characterized by muscle hyperexcitability or weakness which, exacerbated by potassium, heat or cold, can lead to uncontrolled shaking followed by paralysis. Onset usually occurs in early childhood, but it still occurs with adults.

Amiloride, sold under the trade name Midamor among others, is a medication typically used with other medications to treat high blood pressure or swelling due to heart failure or cirrhosis of the liver. Amiloride is classified as a potassium-sparing diuretic. Amiloride is often used together with another diuretic, such as a thiazide or loop diuretic. It is taken by mouth. Onset of action is about two hours and it lasts for about a day.

Indometacin, also known as indomethacin, is a nonsteroidal anti-inflammatory drug (NSAID) commonly used as a prescription medication to reduce fever, pain, stiffness, and swelling from inflammation. It works by inhibiting the production of prostaglandins, endogenous signaling molecules known to cause these symptoms. It does this by inhibiting cyclooxygenase, an enzyme that catalyzes the production of prostaglandins.

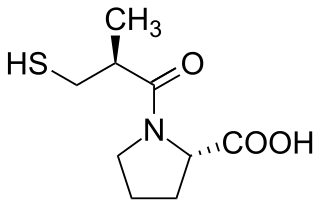
Lisinopril is a medication belonging to the drug class of angiotensin-converting enzyme (ACE) inhibitors and is used to treat high blood pressure, heart failure, and heart attacks. For high blood pressure it is usually a first-line treatment. It is also used to prevent kidney problems in people with diabetes mellitus. Lisinopril is taken by mouth. Full effect may take up to four weeks to occur.
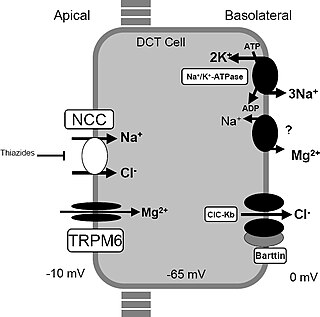
Gitelman syndrome (GS) is an autosomal recessive kidney tubule disorder characterized by low blood levels of potassium and magnesium, decreased excretion of calcium in the urine, and elevated blood pH. It is the most frequent hereditary salt-losing tubulopathy. Gitelman syndrome is caused by disease-causing variants on both alleles of the SLC12A3 gene. The SLC12A3 gene encodes the thiazide-sensitive sodium-chloride cotransporter, which can be found in the distal convoluted tubule of the kidney.

Potassium-sparing diuretics refers to drugs that cause diuresis without causing potassium loss in the urine. They are typically used as an adjunct in management of hypertension, cirrhosis, and congestive heart failure. The steroidal aldosterone antagonists can also be used for treatment of primary hyperaldosteronism. Spironolactone, a steroidal aldosterone antagonist, is also used in management of female hirsutism and acne from PCOS or other causes.
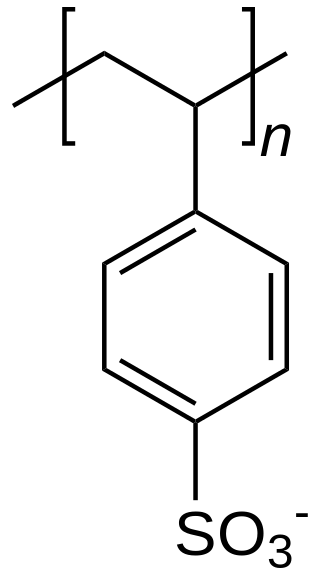
Polystyrene sulfonates are a group of medications used to treat high blood potassium. Effects generally take hours to days. They are also used to remove potassium, calcium, and sodium from solutions in technical applications.
Magnesium deficiency is an electrolyte disturbance in which there is a low level of magnesium in the body. It can result in multiple symptoms. Symptoms include tremor, poor coordination, muscle spasms, loss of appetite, personality changes, and nystagmus. Complications may include seizures or cardiac arrest such as from torsade de pointes. Those with low magnesium often have low potassium.
A mineralocorticoid receptor antagonist or aldosterone antagonist, is a diuretic drug which antagonizes the action of aldosterone at mineralocorticoid receptors. This group of drugs is often used as adjunctive therapy, in combination with other drugs, for the management of chronic heart failure. Spironolactone, the first member of the class, is also used in the management of hyperaldosteronism and female hirsutism. Most antimineralocorticoids, including spironolactone, are steroidal spirolactones. Finerenone is a nonsteroidal antimineralocorticoid.

Eplerenone, sold under the brand name Inspra, is an aldosterone antagonist type of potassium-sparing diuretic that is used to treat chronic heart failure and high blood pressure, particularly for patients with resistant hypertension due to elevated aldosterone. It is a steroidal antimineralocorticoid of the spirolactone group and a selective aldosterone receptor antagonist (SARA). Eplerenone is more selective than spironolactone at the mineralocorticoid receptor relative to binding at androgen, progestogen, glucocorticoid, or estrogen receptors.

Calcium gluconate is the calcium salt of gluconic acid and is used as a mineral supplement and medication. As a medication it is used by injection into a vein to treat low blood calcium, high blood potassium, and magnesium toxicity. Supplementation is generally only required when there is not enough calcium in the diet. Supplementation may be done to treat or prevent osteoporosis or rickets. It can also be taken by mouth but is not recommended for injection into a muscle.
Potassium binders are medications that bind potassium ions in the gastrointestinal tract, thereby preventing its intestinal absorption. This category formerly consisted solely of polystyrene sulfonate, a polyanionic resin attached to a cation, administered either orally or by retention enema to patients who are at risk of developing hyperkalaemia. Newer drugs include: another polyanionic polymer, patiromer, which exchanges calcium for potassium; and Sodium zirconium cyclosilicate crystals, which exchange sodium for potassium

Patiromer, sold under the brand name Veltassa, is a medication used to treat high blood potassium. It is taken by mouth. It works by binding potassium in the gut.

Roxadustat, sold under the brand name Evrenzo, is an anti-anemia medication. Roxadustat is a HIF prolyl-hydroxylase inhibitor that increases endogenous production of erythropoietin and stimulates production of hemoglobin and red blood cells. It was investigated in clinical trials for the treatment of anemia caused by chronic kidney disease (CKD). It is taken by mouth. The drug was developed by FibroGen, in partnership with AstraZeneca.