A lubricant is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, transporting foreign particles, or heating or cooling the surfaces. The property of reducing friction is known as lubricity.

A triglyceride is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils.

Waxes are a diverse class of organic compounds that are lipophilic, malleable solids near ambient temperatures. They include higher alkanes and lipids, typically with melting points above about 40 °C (104 °F), melting to give low viscosity liquids. Waxes are insoluble in water but soluble in nonpolar organic solvents such as hexane, benzene and chloroform. Natural waxes of different types are produced by plants and animals and occur in petroleum.

Motor oil, engine oil, or engine lubricant is any one of various substances used for the lubrication of internal combustion engines. They typically consist of base oils enhanced with various additives, particularly antiwear additives, detergents, dispersants, and, for multi-grade oils, viscosity index improvers. The main function of motor oil is to reduce friction and wear on moving parts and to clean the engine from sludge and varnish (detergents). It also neutralizes acids that originate from fuel and from oxidation of the lubricant (detergents), improves the sealing of piston rings, and cools the engine by carrying heat away from moving parts.
Saponification is a process of cleaving esters into carboxylate salts and alcohols by the action of aqueous alkali. Typically aqueous sodium hydroxide solutions are used. It is an important type of alkaline hydrolysis. When the carboxylate is long chain, its salt is called a soap. The saponification of ethyl acetate gives sodium acetate and ethanol:

The sperm whale or cachalot is the largest of the toothed whales and the largest toothed predator. It is the only living member of the genus Physeter and one of three extant species in the sperm whale family, along with the pygmy sperm whale and dwarf sperm whale of the genus Kogia.

Spermaceti is a waxy substance found in the head cavities of the sperm whale. Spermaceti is created in the spermaceti organ inside the whale's head. This organ may contain as much as 1,900 litres (500 US gal) of spermaceti. It has been extracted by whalers since the 17th century for human use in cosmetics, textiles, and candles.

The melon is a mass of adipose tissue found in the foreheads of all toothed whales. It focuses and modulates the animal's vocalizations and acts as a sound lens. It is thus a key organ involved in communication and echolocation.
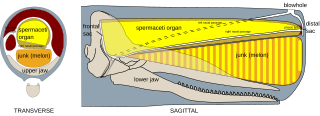
The spermaceti organ is an organ present in the heads of toothed whales of the family Physeteroidea, in particular the sperm whale. This organ contains a waxy liquid called spermaceti and is involved in the generation of sound.

Saponification value or saponification number represents the number of milligrams of potassium hydroxide (KOH) or sodium hydroxide (NaOH) required to saponify one gram of fat under the conditions specified. It is a measure of the average molecular weight of all the fatty acids present in the sample in form of triglycerides. The higher the saponification value, the lower the fatty acids average length, the lighter the mean molecular weight of triglycerides and vice versa. Practically, fats or oils with high saponification value are more suitable for soap making.
Whale oil is oil obtained from the blubber of whales. Oil from the bowhead whale was sometimes known as train-oil, which comes from the Dutch word traan.
Grease is a solid or semisolid lubricant formed as a dispersion of thickening agents in a liquid lubricant. Grease generally consists of a soap emulsified with mineral or vegetable oil.

Jojoba oil is the liquid produced in the seed of the Simmondsia chinensis (jojoba) plant, a shrub, which is native to southern Arizona, southern California, and northwestern Mexico. The oil makes up approximately 50% of the jojoba seed by weight. The terms "jojoba oil" and "jojoba wax" are often used interchangeably because the wax visually appears to be a mobile oil, but as a wax it is composed almost entirely (~97%) of mono-esters of long-chain fatty acids (wax ester) and alcohols, accompanied by only a tiny fraction of triglyceride esters. This composition accounts for its extreme shelf-life stability and extraordinary resistance to high temperatures, compared with true vegetable oils.
Jojoba esters are the hydrogenation or transesterification product of Jojoba oil. Jojoba Esters are commonly used in cosmetic formulations as an emollient, due to its remarkable similarity to the natural oils produced by the human skin, and its high oxidative stability. Fully hydrogenated jojoba esters are most often small beads used to exfoliate the skin.
An oil is any nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobic and lipophilic. Oils are usually flammable and surface active. Most oils are unsaturated lipids that are liquid at room temperature.
A wax ester (WE) is an ester of a fatty acid and a fatty alcohol. Wax esters are the main components of three commercially important waxes: carnauba wax, candelilla wax, and beeswax.
Oil additives are chemical compounds that improve the lubricant performance of base oil. The manufacturer of many different oils can utilize the same base stock for each formulation and can choose different additives for each specific application. Additives comprise up to 5% by weight of some oils.
Winterizationof oil is a process that uses a solvent and cold temperatures to separate lipids and other desired oil compounds from waxes. Winterization is a type of fractionation, the general process of separating the triglycerides found in fats and oils, using the difference in their melting points, solubility, and volatility.

Sperm whaling is the human practice of hunting sperm whales, the largest toothed whale and the deepest-diving marine mammal species, for the oil, meat and bone that can be extracted from the cetaceans' bodies.

A defoamer or an anti-foaming agent is a chemical additive that reduces and hinders the formation of foam in industrial process liquids. The terms anti-foam agent and defoamer are often used interchangeably. Strictly speaking, defoamers eliminate existing foam and anti-foamers prevent the formation of further foam. Commonly used agents are insoluble oils, polydimethylsiloxanes and other silicones, certain alcohols, stearates and glycols. The additive is used to prevent formation of foam or is added to break a foam already formed.


















