
Glucocorticoids are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every vertebrate animal cell. The name "glucocorticoid" is a portmanteau and is composed from its role in regulation of glucose metabolism, synthesis in the adrenal cortex, and its steroidal structure.

Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system of vertebrates– a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands: endocannabinoids; phytocannabinoids ; and synthetic cannabinoids. All endocannabinoids and phytocannabinoids are lipophilic.

A lipoxin (LX or Lx), an acronym for lipoxygenase interaction product, is a bioactive autacoid metabolite of arachidonic acid made by various cell types. They are categorized as nonclassic eicosanoids and members of the specialized pro-resolving mediators (SPMs) family of polyunsaturated fatty acid (PUFA) metabolites. Like other SPMs, LXs form during, and then act to resolve, inflammatory responses. Initially, two lipoxins were identified, lipoxin A4 (LXA4) and LXB4, but more recent studies have identified epimers of these two LXs: the epi-lipoxins, 15-epi-LXA4 and 15-epi-LXB4 respectively.
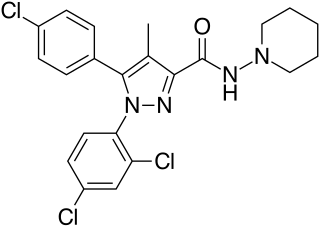
Rimonabant (also known as SR141716; trade names Acomplia, Zimulti) is an anorectic antiobesity drug approved in Europe in 2006 but was withdrawn worldwide in 2008 due to serious psychiatric side effects; it was never approved in the United States. Rimonabant is an inverse agonist for the cannabinoid receptor CB1 and was first-in-class for clinical development.
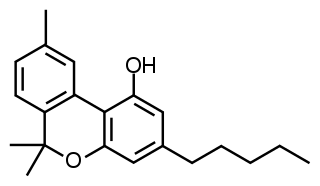
Cannabinol (CBN) is a mildly psychoactive cannabinoid that acts as a low affinity partial agonist at both CB1 and CB2 receptors. This activity at CB1 and CB2 receptors constitutes interaction of CBN with the endocannabinoid system (ECS).

Trichostatin A (TSA) is an organic compound that serves as an antifungal antibiotic and selectively inhibits the class I and II mammalian histone deacetylase (HDAC) families of enzymes, but not class III HDACs. However, there are recent reports of the interactions of this molecule with Sirt 6 protein. TSA inhibits the eukaryotic cell cycle during the beginning of the growth stage. TSA can be used to alter gene expression by interfering with the removal of acetyl groups from histones and therefore altering the ability of DNA transcription factors to access the DNA molecules inside chromatin. It is a member of a larger class of histone deacetylase inhibitors that have a broad spectrum of epigenetic activities. Thus, TSA has some potential as an anti-cancer drug. One suggested mechanism is that TSA promotes the expression of apoptosis-related genes, leading to cancerous cells surviving at lower rates, thus slowing the progression of cancer. Other mechanisms may include the activity of HDIs to induce cell differentiation, thus acting to "mature" some of the de-differentiated cells found in tumors. HDIs have multiple effects on non-histone effector molecules, so the anti-cancer mechanisms are truly not understood at this time.

WIN 55,212-2 is a chemical described as an aminoalkylindole derivative, which produces effects similar to those of cannabinoids such as tetrahydrocannabinol (THC) but has an entirely different chemical structure.

The endocannabinoid system (ECS) is a biological system composed of endocannabinoids, which are endogenous lipid-based retrograde neurotransmitters that bind to cannabinoid receptors, and cannabinoid receptor proteins that are expressed throughout the vertebrate central nervous system and peripheral nervous system. The endocannabinoid system remains under preliminary research, but may be involved in regulating physiological and cognitive processes, including fertility, pregnancy, pre- and postnatal development, various activity of immune system, appetite, pain-sensation, mood, and memory, and in mediating the pharmacological effects of cannabis. The ECS plays an important role in multiple aspects of neural functions, including the control of movement and motor coordination, learning and memory, emotion and motivation, addictive-like behavior and pain modulation, among others.

The transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the TRPV1 gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins that in turn are a sub-family of the transient receptor potential protein group. This protein is a member of the TRPV group of transient receptor potential family of ion channels. Fatty acid metabolites with affinity for this receptor are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA). The function of TRPV1 is detection and regulation of body temperature. In addition, TRPV1 provides a sensation of scalding heat and pain (nociception). In primary afferent sensory neurons, it cooperates with TRPA1 to mediate the detection of noxious environmental stimuli.

AM404, also known as N-arachidonoylphenolamine, is an active metabolite of paracetamol (acetaminophen), responsible for all or part of its analgesic action and anticonvulsant effects. Chemically, it is the amide formed from 4-aminophenol and arachidonic acid.

Cannabinoid receptor 1 (CB1), is a G protein-coupled cannabinoid receptor that in humans is encoded by the CNR1 gene. The human CB1 receptor is expressed in the peripheral nervous system and central nervous system. It is activated by endocannabinoids, a group of retrograde neurotransmitters that include anandamide and 2-arachidonoylglycerol (2-AG); plant phytocannabinoids, such as docosatetraenoylethanolamide found in wild daga, the compound THC which is an active constituent of the psychoactive drug cannabis; and synthetic analogs of THC. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin (THCV).

The cannabinoid receptor 2(CB2), is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the CNR2 gene. It is closely related to the cannabinoid receptor 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids. The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG).

N-Arachidonoyl dopamine (NADA) is an endocannabinoid that acts as an agonist of the CB1 receptor and the transient receptor potential V1 (TRPV1) ion channel. NADA was first described as a putative endocannabinoid (agonist for the CB1 receptor) in 2000 and was subsequently identified as an endovanilloid (agonist for TRPV1) in 2002. NADA is an endogenous arachidonic acid based lipid found in the brain of rats, with especially high concentrations in the hippocampus, cerebellum, and striatum. It activates the TRPV1 channel with an EC50 of approximately of 50 nM which makes it the putative endogenous TRPV1 agonist.

An N-acylethanolamine (NAE) is a type of fatty acid amide where one of several types of acyl groups is linked to the nitrogen atom of ethanolamine, and highly metabolic formed by intake of essential fatty acids through diet by 20:4, n-6 and 22:6, n-3 fatty acids, and when the body is physically and psychologically active,. The endocannabinoid signaling system (ECS) is the major pathway by which NAEs exerts its physiological effects in animal cells with similarities in plants, and the metabolism of NAEs is an integral part of the ECS, a very ancient signaling system, being clearly present from the divergence of the protostomian/deuterostomian, and even further back in time, to the very beginning of bacteria, the oldest organisms on Earth known to express phosphatidylethanolamine, the precursor to endocannabinoids, in their cytoplasmic membranes. Fatty acid metabolites with affinity for CB receptors are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA), by brown algae which diverged about 1500 MYA, by sponges, which diverged from eumetazoans about 930 MYA, and a lineages that predate the evolution of CB receptors, as CB1 – CB2 duplication event may have occurred prior to the lophotrochozoan-deuterostome divergence 590 MYA. Fatty acid amide hydrolase (FAAH) evolved relatively recently, either after the evolution of fish 400 MYA, or after the appearance of mammals 300 MYA, but after the appearance of vertebrates. Linking FAAH, vanilloid receptors (VR1) and anandamide implies a coupling among the remaining ‘‘older’’ parts of the endocannabinoid system, monoglyceride lipase (MGL), CB receptors, that evolved prior to the metazoan-bilaterian divergence, but were secondarily lost in the Ecdysozoa, and 2-Arachidonoylglycerol (2-AG).
Palmitoylethanolamide (PEA) is an endogenous fatty acid amide, and lipid modulator PEA has been studied in in vitro and in vivo systems using exogenously added or dosed compound; there is evidence that it binds to a nuclear receptor, through which it exerts a variety of biological effects, some related to chronic inflammation and pain.

Acmella oleracea is a species of flowering herb in the family Asteraceae. Common names include toothache plant, Szechuan buttons, paracress, jambu, buzz buttons, tingflowers and electric daisy. Its native distribution is unclear, but it is likely derived from a Brazilian Acmella species. A small, erect plant, it grows quickly and bears gold and red inflorescences. It is frost-sensitive but perennial in warmer climates.

LASSBio-881 is a drug which acts as both a non-selective partial agonist of the CB1 and CB2 cannabinoid receptors, and also as an antagonist of the TRPV1 receptor, as well as having antioxidant effects. It has potent anti-inflammatory and anti-hyperalgesic effects in animal studies.

Tetrahydrocannabinolic acid is a precursor of tetrahydrocannabinol (THC), an active component of cannabis.
Regulatory macrophages (Mregs) represent a subset of anti-inflammatory macrophages. In general, macrophages are a very dynamic and plastic cell type and can be divided into two main groups: classically activated macrophages (M1) and alternatively activated macrophages (M2). M2 group can further be divided into sub-groups M2a, M2b, M2c, and M2d. Typically the M2 cells have anti-inflammatory and regulatory properties and produce many different anti-inflammatory cytokines such as IL-4, IL-33, IL-10, IL-1RA, and TGF-β. M2 cells can also secrete angiogenic and chemotactic factors. These cells can be distinguished based on the different expression levels of various surface proteins and the secretion of different effector molecules.
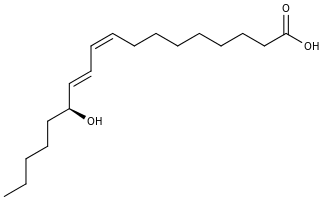
13-Hydroxyoctadecadienoic acid (13-HODE) is the commonly used term for 13(S)-hydroxy-9Z,11E-octadecadienoic acid (13(S)-HODE). The production of 13(S)-HODE is often accompanied by the production of its stereoisomer, 13(R)-hydroxy-9Z,11E-octadecadienoic acid (13(R)-HODE). The adjacent figure gives the structure for the (S) stereoisomer of 13-HODE. Two other naturally occurring 13-HODEs that may accompany the production of 13(S)-HODE are its cis-trans (i.e., 9E,11E) isomers viz., 13(S)-hydroxy-9E,11E-octadecadienoic acid (13(S)-EE-HODE) and 13(R)-hydroxy-9E,11E-octadecadienoic acid (13(R)-EE-HODE). Studies credit 13(S)-HODE with a range of clinically relevant bioactivities; recent studies have assigned activities to 13(R)-HODE that differ from those of 13(S)-HODE; and other studies have proposed that one or more of these HODEs mediate physiological and pathological responses, are markers of various human diseases, and/or contribute to the progression of certain diseases in humans. Since, however, many studies on the identification, quantification, and actions of 13(S)-HODE in cells and tissues have employed methods that did not distinguish between these isomers, 13-HODE is used here when the actual isomer studied is unclear.


















