
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals, are coordination complexes.
In chemistry, a transition metal is a chemical element in the d-block of the periodic table, though the elements of group 12 are sometimes excluded. The lanthanide and actinide elements are called inner transition metals and are sometimes considered to be transition metals as well.

Magnetic circular dichroism (MCD) is the differential absorption of left and right circularly polarized (LCP and RCP) light, induced in a sample by a strong magnetic field oriented parallel to the direction of light propagation. MCD measurements can detect transitions which are too weak to be seen in conventional optical absorption spectra, and it can be used to distinguish between overlapping transitions. Paramagnetic systems are common analytes, as their near-degenerate magnetic sublevels provide strong MCD intensity that varies with both field strength and sample temperature. The MCD signal also provides insight into the symmetry of the electronic levels of the studied systems, such as metal ion sites.
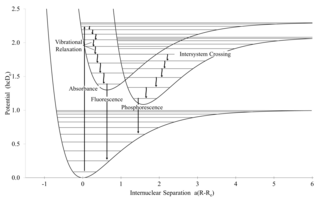
Intersystem crossing (ISC) is an isoenergetic radiationless process involving a transition between the two electronic states with different spin multiplicity.
In molecular physics, crystal field theory (CFT) describes the breaking of degeneracies of electron orbital states, usually d or f orbitals, due to a static electric field produced by a surrounding charge distribution. This theory has been used to describe various spectroscopies of transition metal coordination complexes, in particular optical spectra (colors). CFT successfully accounts for some magnetic properties, colors, hydration enthalpies, and spinel structures of transition metal complexes, but it does not attempt to describe bonding. CFT was developed by physicists Hans Bethe and John Hasbrouck van Vleck in the 1930s. CFT was subsequently combined with molecular orbital theory to form the more realistic and complex ligand field theory (LFT), which delivers insight into the process of chemical bonding in transition metal complexes. CFT can be complicated further by breaking assumptions made of relative metal and ligand orbital energies, requiring the use of inverted ligand field theory (ILFT) to better describe bonding.

Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula Fe(CO)5. Under standard conditions Fe(CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to diverse iron compounds, including many that are useful in small scale organic synthesis.
A spectrochemical series is a list of ligands ordered by ligand "strength", and a list of metal ions based on oxidation number, group and element. For a metal ion, the ligands modify the difference in energy Δ between the d orbitals, called the ligand-field splitting parameter in ligand field theory, or the crystal-field splitting parameter in crystal field theory. The splitting parameter is reflected in the ion's electronic and magnetic properties such as its spin state, and optical properties such as its color and absorption spectrum.
Iron shows the characteristic chemical properties of the transition metals, namely the ability to form variable oxidation states differing by steps of one and a very large coordination and organometallic chemistry: indeed, it was the discovery of an iron compound, ferrocene, that revolutionalized the latter field in the 1950s. Iron is sometimes considered as a prototype for the entire block of transition metals, due to its abundance and the immense role it has played in the technological progress of humanity. Its 26 electrons are arranged in the configuration [Ar]3d64s2, of which the 3d and 4s electrons are relatively close in energy, and thus it can lose a variable number of electrons and there is no clear point where further ionization becomes unprofitable.

In chemistry and physics, LIESST is a method of changing the electronic spin state of a compound by means of irradiation with light.
The spin transition is an example of transition between two electronic states in molecular chemistry. The ability of an electron to transit from a stable to another stable electronic state in a reversible and detectable fashion, makes these molecular systems appealing in the field of molecular electronics.
Spin states when describing transition metal coordination complexes refers to the potential spin configurations of the central metal's d electrons. For several oxidation states, metals can adopt high-spin and low-spin configurations. The ambiguity only applies to first row metals, because second- and third-row metals are invariably low-spin. These configurations can be understood through the two major models used to describe coordination complexes; crystal field theory and ligand field theory.
Sulfur mononitride is an inorganic compound with the molecular formula SN. It is the sulfur analogue of and isoelectronic to the radical nitric oxide, NO. It was initially detected in 1975, in outer space in giant molecular clouds and later the coma of comets. This spurred further laboratory studies of the compound. Synthetically, it is produced by electric discharge in mixtures of nitrogen and sulfur compounds, or combustion in the gas phase and by photolysis in solution.
Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or more unpaired electrons are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metals the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin–orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanides and actinides, spin–orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism, antiferromagnetism or ferrimagnetism depending on the relative orientations of the individual spins.

Charge-transfer bands are a characteristic feature of the optical spectra of many compounds. These bands are typically more intense than d–d transitions. They typically exhibit solvatochromism, consistent with shifts of electron density that would be sensitive to solvation.

Iron tris(dimethyldithiocarbamate) is the coordination complex of iron with dimethyldithiocarbamate with the formula Fe(S2CNMe2)3 (Me = methyl). It is marketed as a fungicide.
OctaDist is computer software for crystallography and inorganic chemistry program. It is mainly used for computing distortion parameters of coordination complex such as spin crossover complex (SCO), magnetic metal complex and metal–organic framework (MOF).

2-Picolylamine is an organic compound with the formula H2NCH2C5H4N. A colorless liquid, it is a common bidentate ligand and a precursor to more complex multidentate ligands such as tris(2-pyridylmethyl)amine. It is usually prepared by hydrogenation of 2-cyanopyridine. One such complex is Baratta's catalyst RuCl2(PPh3)2(ampy) (ampy = 2-picolylamine) for transfer hydrogenation. Salts of the complex [Fe(pyCH2NH2)3]2+ exhibit spin crossover behavior, whereby the complex switches from high to low spin configurations, depending on the temperature.
Azzedine Bousseksou is a Franco Algerian physical chemist.

Mixed valence complexes contain an element which is present in more than one oxidation state. Well-known mixed valence compounds include the Creutz–Taube complex, Prussian blue, and molybdenum blue. Many solids are mixed-valency including indium chalcogenides.

Iron tris(diethyldithiocarbamate) is the coordination complex of iron with diethyldithiocarbamate with the formula Fe(S2CNEt2)3 (Et = ethyl). It is a black solid that is soluble in organic solvents.














