
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and may require a mordant to improve the fastness of the dye on the fiber.

Thiamine, also known as thiamin and vitamin B1, is a vitamin, an essential micronutrient for humans and animals. It is found in food and commercially synthesized to be a dietary supplement or medication. Phosphorylated forms of thiamine are required for some metabolic reactions, including the breakdown of glucose and amino acids.

In biochemistry, cellulose acetate refers to any acetate ester of cellulose, usually cellulose diacetate. It was first prepared in 1865. A bioplastic, cellulose acetate is used as a film base in photography, as a component in some coatings, and as a frame material for eyeglasses; it is also used as a synthetic fiber in the manufacture of cigarette filters and playing cards. In photographic film, cellulose acetate film replaced nitrate film in the 1950s, being far less flammable and cheaper to produce.

Lead(II) chloride (PbCl2) is an inorganic compound which is a white solid under ambient conditions. It is poorly soluble in water. Lead(II) chloride is one of the most important lead-based reagents. It also occurs naturally in the form of the mineral cotunnite.
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.
A tetrahedral intermediate is a reaction intermediate in which the bond arrangement around an initially double-bonded carbon atom has been transformed from trigonal to tetrahedral. Tetrahedral intermediates result from nucleophilic addition to a carbonyl group. The stability of tetrahedral intermediate depends on the ability of the groups attached to the new tetrahedral carbon atom to leave with the negative charge. Tetrahedral intermediates are very significant in organic syntheses and biological systems as a key intermediate in esterification, transesterification, ester hydrolysis, formation and hydrolysis of amides and peptides, hydride reductions, and other chemical reactions.

Crotonic acid ((2E)-but-2-enoic acid) is a short-chain unsaturated carboxylic acid described by the formula CH3CH=CHCO2H. The name crotonic acid was given because it was erroneously thought to be a saponification product of croton oil. It crystallizes as colorless needles from hot water. With a cis-alkene, Isocrotonic acid is an isomer of crotonic acid. Crotonic acid is soluble in water and many organic solvents. Its odor is similar to that of butyric acid.

Vanadium(III) chloride describes the inorganic compound with the formula VCl3 and its hydrates. It forms a purple anhydrous form and a green hexahydrate [VCl2(H2O)4]Cl·2H2O. These hygroscopic salts are common precursors to other vanadium(III) complexes and is used as a mild reducing agent.

The tert-butyloxycarbonyl protecting group or tert-butoxycarbonyl protecting group is a protecting group used in organic synthesis.
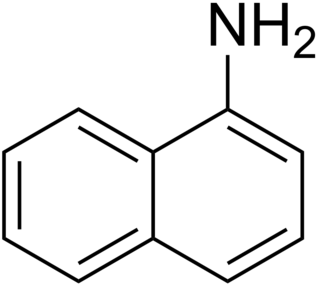
1-Naphthylamine is an aromatic amine derived from naphthalene. It can cause bladder cancer. It crystallizes in colorless needles which melt at 50 °C. It possesses a disagreeable odor, sublimes readily, and turns brown on exposure to air. It is the precursor to a variety of dyes.

Dihydroxymalonic acid is an organic compound with formula C3H4O6 or HO-(C=O)-C(OH)2-(C=O)-OH, found in some plants such as alfalfa and in beet molasses.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC.
Zinc compounds are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table. The oxidation state of zinc in most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element with zinc(II). Zinc compounds are noteworthy for their nondescript appearance and behavior: they are generally colorless, do not readily engage in redox reactions, and generally adopt symmetrical structures.

Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hormone therapy, in the treatment of gynecological disorders, and in the treatment of androgen-dependent conditions like enlarged prostate and prostate cancer in men and acne and hirsutism in women. It is available both at a low dose in combination with an estrogen in birth control pills and, in a few countries like France and Japan, at low, moderate, and high doses alone for various indications. It is taken by mouth.

Tetrahydroxy-1,4-benzoquinone, also called tetrahydroxy-p-benzoquinone, tetrahydroxybenzoquinone, or tetrahydroxyquinone, is an organic compound with formula C6O2(OH)4. Its molecular structure consists of a cyclohexadiene ring with four hydroxyl groups and two ketone groups in opposite (para) positions.

Spinochrome E is a polyhydroxylated 1,4-naphthoquinone pigment found in sea urchin shell ("test"), spine, gonads, coelomic fluid, and eggs, of sea urchin commonly known as spinochromes. These natural phenolic compounds are quinones with potential pharmacological properties. The several hydroxyl groups are appropriate for free-radical scavenging, which diminishes ROS and prevents redox imbalance. Mechanisms are described such as scavenging of reactive oxygen species (ROS), interaction with lipid peroxide radicals, chelation of metal ions, inhibition of lipid peroxidation and regulation of the cell redox potential.

Spinochrome D (2,3,5,6,8-pentahydroxy-1,4-naphthoquinone) is an organic compound with formula C
10H
6O
5, formally derived from 1,4-naphthoquinone through the replacement of five hydrogen atoms by hydroxyl (OH) groups.

Toxopneustes pileolus, commonly known as the flower urchin, is a widespread and commonly encountered species of sea urchin from the Indo-West Pacific. It is considered highly dangerous, as it is capable of delivering extremely painful and medically significant stings when touched. It inhabits coral reefs, seagrass beds, and rocky or sandy environments at depths of up to 90 m (295 ft). It feeds on algae, bryozoans, and organic detritus.

Chika Kuroda was a Japanese chemist whose research focused on natural pigments. She was the first woman in Japan to receive a Bachelor of Science.

Fallacinol (teloschistin) is an organic compound in the structural class of chemicals known as anthraquinones. It is found in some lichens, particularly in the family Teloschistaceae, as well as a couple of plants and non lichen-forming fungi. In 1936, Japanese chemists isolated a pigment named fallacin from the lichen Oxneria fallax, which was later refined and assigned a tentative structural formula; by 1949, Indian chemists had isolated a substance from Teloschistes flavicans with an identical structural formula to fallacin. Later research further separated fallacin into two distinct pigments, fallacin-A and fallacin-B (fallacinol). The latter compound is also known as teloschistin due to its structural match with the substance isolated earlier.


















