
A metallocene is a compound typically consisting of two cyclopentadienyl anions (C
5H−
5, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula (C5H5)2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze olefin polymerization.
Ferrocene is an organometallic compound with the formula Fe(C
5H
5)
2. The molecule consists of two cyclopentadienyl rings bound on opposite sides of a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C
5H
5)+
2.

Nickelocene is the organonickel compound with the formula Ni(η5-C5H5)2. Also known as bis(cyclopentadienyl)nickel or NiCp2, this bright green paramagnetic solid is of enduring academic interest, although it does not yet have any known practical applications.
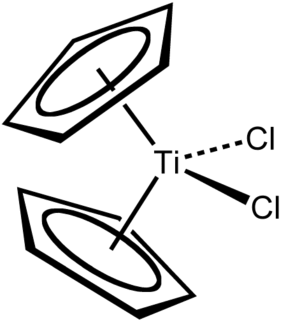
Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.
Vanadocene dichloride is an organometallic complex with formula (η5-C5H5)2VCl2 (commonly abbreviated as Cp2VCl2). It is a structural analogue of titanocene dichloride but with vanadium(IV) instead of titanium(IV). This compound has one unpaired electron, hence Cp2VCl2 is paramagnetic. Vanadocene dichloride is a suitable precursor for variety of bis(cyclopentadienyl)vanadium(IV) compounds.

Hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated. In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity, and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands.
Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:

Organoactinide chemistry is the science exploring the properties, structure and reactivity of organoactinide compounds, which are organometallic compounds containing a carbon to actinide chemical bond.

Organotitanium compounds in organometallic chemistry contain carbon-to-titanium chemical bonds. Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis and reactions. They are reagents in organic chemistry and are involved in major industrial processes.
Niobocene dichloride is the organometallic compound with the formula (C5H5)2NbCl2, abbreviated Cp2NbCl2. This paramagnetic brown solid is a starting reagent for the synthesis of other organoniobium compounds. The compound adopts a pseudotetrahedral structure with two cyclopentadienyl and two chloride substituents attached to the metal. A variety of similar compounds are known, including Cp2TiCl2.
In organometallic chemistry, a transition metal indenyl complex is a coordination compound that contains one or more indenyl ligands. The indenyl ligand is formally the anion derived from deprotonation of indene. The η5-indenyl ligand is related to the η5cyclopentadienyl anion (Cp), thus indenyl analogues of many cyclopentadienyl complexes are known. Indenyl ligands lack the 5-fold symmetry of Cp, so they exhibit more complicated geometries. Furthermore, some indenyl complexes also exist with only η3-bonding mode. The η5- and η3-bonding modes sometimes interconvert.
Zirconocene dichloride is an organozirconium compound composed of a zirconium central atom, with two cyclopentadienyl and two chloro ligands. It is a colourless diamagnetic solid that is somewhat stable in air.

Cyclopentadienylcobalt dicarbonyl is an organocobalt compound with formula (C5H5)Co(CO)2, abbreviated CpCo(CO)2. It is an example of a half-sandwich complex. It is a dark red air sensitive liquid. This compound features one cyclopentadienyl ring that is bound in an η5-manner and two carbonyl ligands. The compound is soluble in common organic solvents.

Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium is the organoruthenium half-sandwich compound with formula RuCl(PPh3)2(C5H5). It as an air-stable orange crystalline solid that is used in a variety of organometallic synthetic and catalytic transformations. The compound has idealized Cs symmetry. It is soluble in chloroform, dichloromethane, and acetone.
Organoscandium chemistry is the chemistry of organometallic compounds containing a carbon to scandium chemical bond. The interest in organoscandium compounds is mostly academic but several compound classes find practical application in catalysis, especially in polymerization. A common precursor is scandium chloride.
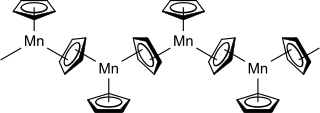
Manganocene or bis(cyclopentadienyl)manganese(II) is an organomanganese compound with the formula [Mn(C5H5)2]n. It is a thermochromic solid that degrades rapidly in air. Although the compound is of little utility, it is often discussed as an example of a metallocene with ionic character.

Metal bis(trimethylsilyl)amides are coordination complexes composed of a cationic metal with anionic bis(trimethylsilyl)amide ligands and are part of a broader category of metal amides.
Molybdocene dichloride is the organomolybdenum compound with the formula (η5-C5H5)2MoCl2 and IUPAC name dichlorobis(η5-cyclopentadienyl)molybdenum(IV), and is commonly abbreviated as Cp2MoCl2. It is a brownish-green air- and moisture-sensitive powder. In the research laboratory, it is used to prepare many derivatives.
f-Block Metallocene refers to a class of organometallic sandwich compounds with f-Block metal and electron-rich ligands like cyclopentadienyl anion.

A lanthanocene is a type of metallocene compound that contains an element from the lanthanide series. The most common lanthanocene complexes contain two cyclopentadienyl anions and an X type ligand, usually hydride or alkyl ligand.











