Related Research Articles

Gene therapy is a medical technology which aims to produce a therapeutic effect through the manipulation of gene expression or through altering the biological properties of living cells.

In multicellular organisms, stem cells are undifferentiated or partially differentiated cells that can differentiate into various types of cells and proliferate indefinitely to produce more of the same stem cell. They are the earliest type of cell in a cell lineage. They are found in both embryonic and adult organisms, but they have slightly different properties in each. They are usually distinguished from progenitor cells, which cannot divide indefinitely, and precursor or blast cells, which are usually committed to differentiating into one cell type.

Multiple myeloma (MM), also known as plasma cell myeloma and simply myeloma, is a cancer of plasma cells, a type of white blood cell that normally produces antibodies. Often, no symptoms are noticed initially. As it progresses, bone pain, anemia, kidney dysfunction, and infections may occur. Complications may include hypercalcemia and amyloidosis.

Embryonic stem cells (ESCs) are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre-implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells. Isolating the inner cell mass (embryoblast) using immunosurgery results in destruction of the blastocyst, a process which raises ethical issues, including whether or not embryos at the pre-implantation stage have the same moral considerations as embryos in the post-implantation stage of development.
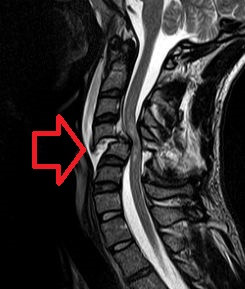
A spinal cord injury (SCI) is damage to the spinal cord that causes temporary or permanent changes in its function. Symptoms may include loss of muscle function, sensation, or autonomic function in the parts of the body served by the spinal cord below the level of the injury. Injury can occur at any level of the spinal cord and can be complete, with a total loss of sensation and muscle function at lower sacral segments, or incomplete, meaning some nervous signals are able to travel past the injured area of the cord up to the Sacral S4-5 spinal cord segments. Depending on the location and severity of damage, the symptoms vary, from numbness to paralysis, including bowel or bladder incontinence. Long term outcomes also range widely, from full recovery to permanent tetraplegia or paraplegia. Complications can include muscle atrophy, loss of voluntary motor control, spasticity, pressure sores, infections, and breathing problems.
Cord blood is blood that remains in the placenta and in the attached umbilical cord after childbirth. Cord blood is collected because it contains stem cells, which can be used to treat hematopoietic and genetic disorders such as cancer. There is growing interest from cell therapeutics companies in developing genetically modified allogenic natural killer cells from umbilical cord blood as an alternative to CAR T cell therapies for rare diseases.

Geron Corporation is a biotechnology company located in Foster City, California, which specializes in developing and commercializing therapeutic products for cancer that inhibit telomerase.
Stem-cell therapy is the use of stem cells to treat or prevent a disease or condition. As of 2016, the only established therapy using stem cells is hematopoietic stem cell transplantation. This usually takes the form of a bone-marrow transplantation, but the cells can also be derived from umbilical cord blood. Research is underway to develop various sources for stem cells as well as to apply stem-cell treatments for neurodegenerative diseases and conditions such as diabetes and heart disease.

A spinal cord stimulator (SCS) or dorsal column stimulator (DCS) is a type of implantable neuromodulation device that is used to send electrical signals to select areas of the spinal cord for the treatment of certain pain conditions. SCS is a consideration for people who have a pain condition that has not responded to more conservative therapy. There are also spinal cord stimulators under research and development that could enable patients with spinal cord injury to walk again via epidural electrical stimulation (EES).

Spinal muscular atrophy (SMA) is a rare neuromuscular disorder that results in the loss of motor neurons and progressive muscle wasting. It is usually diagnosed in infancy or early childhood and if left untreated it is the most common genetic cause of infant death. It may also appear later in life and then have a milder course of the disease. The common feature is progressive weakness of voluntary muscles, with arm, leg and respiratory muscles being affected first. Associated problems may include poor head control, difficulties swallowing, scoliosis, and joint contractures.
Mecasermin rinfabate, also known as rhIGF-1/rhIGFBP-3, is a drug consisting of recombinant human insulin-like growth factor 1 (IGF-1) and recombinant human insulin-like growth factor binding protein-3 (IGFBP-3) which is used for the treatment of amyotrophic lateral sclerosis.
Sipuleucel-T, sold under the brand name Provenge, developed by Dendreon Pharmaceuticals, LLC, is a cell-based cancer immunotherapy for prostate cancer (CaP). It is an autologous cellular immunotherapy.

Spinal stenosis is an abnormal narrowing of the spinal canal or neural foramen that results in pressure on the spinal cord or nerve roots. Symptoms may include pain, numbness, or weakness in the arms or legs. Symptoms are typically gradual in onset and improve with leaning forward. Severe symptoms may include loss of bladder control, loss of bowel control, or sexual dysfunction.

Nivolumab, sold under the brand name Opdivo, is a medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction (GEJ) cancer. It is used by slow injection into a vein.
The Food and Drug Administration (FDA) approved the first clinical trial in the United States involving human embryonic stem cells on January 23, 2009. Geron Corporation, a biotechnology firm located in Menlo Park, California, originally planned to enroll ten patients with spinal cord injuries to participate in the trial. The company hoped that GRNOPC1, a product derived from human embryonic stem cells, would stimulate nerve growth in patients with debilitating damage to the spinal cord. The trial began in 2010 after being delayed by the FDA because cysts were found on mice injected with these cells, and safety concerns were raised.
Paul S. Knoepfler is an American biologist, writer, and blogger. He is a professor in the Department of Cell Biology and Human Anatomy, the Genome Center, and the Comprehensive Cancer Center at the University of California, Davis School of Medicine. In 2013, Knoepfler was named one of the 50 most influential people in the stem cell field.

United States of America v. Regenerative Sciences, LLC, 741 F.3d 1314, was a decision in the United States Court of Appeals for the District of Columbia Circuit filed on February 4, 2014 concerning more than minimally manipulated cell therapies and whether they are considered part of medical practice or a drug, the latter subjecting it to regulation under the Food and Drug Administration (FDA). Regenerative Sciences LLC marketed a therapy procedure called Regenexx-C for the treatment of arthritis and orthopedic injury that involved extraction and culture of mesenchymal stem cells from the same patient which were later reinjected. In 2008, The FDA notified Regenerative Sciences LLC that the procedure may not be in compliance with their regulation using an Untitled Letter, which began a series of suits and counter suits, leading to the 2014 decision upholding the FDA’s regulation of more than minimally manipulated stem cell therapies.
Spinal cord injury research seeks new ways to cure or treat spinal cord injury in order to lessen the debilitating effects of the injury in the short or long term. There is no cure for SCI, and current treatments are mostly focused on spinal cord injury rehabilitation and management of the secondary effects of the condition. Two major areas of research include neuroprotection, ways to prevent damage to cells caused by biological processes that take place in the body after the injury, and neuroregeneration, regrowing or replacing damaged neural circuits.
Young blood transfusion refers to transfusing blood specifically from a young person into an older one with the intention of creating a health benefit. The scientific community currently views the practice as essentially pseudoscientific, with comparisons to snake oil. There are also concerns of harm. The U.S. Food and Drug Administration, in 2019, cautioned "consumers against receiving young donor plasma infusions" stating that they are an "unproven treatment".
Advanced Cell Therapeutics was a company that marketed fraudulent medical treatments in the form of stem cell therapy, starting in 2002. It was originally located in Atlanta, and offered its services in the US and Europe, and was founded and run by Stephen van Rooyen and Laura Brown. After the US Food and Drug Administration closed the company down, van Rooyen and Brown fled to South Africa and started operating the business under the name Advanced Cell Therapeutics.
References
- ↑ Bauer, Gerhard; Elsallab, Magdi; Abou-El-Enein, Mohamed (September 10, 2018). "Concise Review: A Comprehensive Analysis of Reported Adverse Events in Patients Receiving Unproven Stem Cell-Based Interventions". Stem Cells Translational Medicine. 7 (9): 676–685. doi:10.1002/sctm.17-0282. PMC 6127222 . PMID 30063299.
- ↑ Turner, L. (2015). "US stem cell clinics, patient safety, and the FDA". Trends in Molecular Medicine. 21 (5): 271–3. doi:10.1016/j.molmed.2015.02.008. PMID 25945404.
- ↑ Knoepfler, P. S. (2015). "From bench to FDA to bedside: US regulatory trends for new stem cell therapies". Advanced Drug Delivery Reviews. 82–83: 192–6. doi:10.1016/j.addr.2014.12.001. PMC 4398607 . PMID 25489841.
- ↑ Knoepfler, P. S. (2019). "Rapid change of a cohort of 570 unproven stem cell clinics in the USA over 3 years". Regenerative Medicine. 14 (8): 735–740. doi: 10.2217/rme-2019-0064 . PMID 31456478.
- ↑ Berkowitz, Aaron L.; Miller, Michael B.; Mir, Saad A.; Cagney, Daniel; Chavakula, Vamsidhar; Guleria, Indira; Aizer, Ayal; Ligon, Keith L.; Chi, John H. (July 14, 2016). "Glioproliferative Lesion of the Spinal Cord as a Complication of "Stem-Cell Tourism"". The New England Journal of Medicine. 375 (2): 196–198. doi:10.1056/NEJMc1600188. PMID 27331440.
- ↑ Berkowitz, Aaron L.; Miller, Michael B.; Mir, Saad A.; Cagney, Daniel; Chavakula, Vamsidhar; Guleria, Indira; Aizer, Ayal; Ligon, Keith L.; Chi, John H. (July 14, 2016). "Glioproliferative Lesion of the Spinal Cord as a Complication of "Stem-Cell Tourism"". The New England Journal of Medicine. 375 (2): 196–198. doi:10.1056/NEJMc1600188. PMID 27331440.
- ↑ Bowman, Michelle; Racke, Michael; Kissel, John; Imitola, Jaime (November 10, 2015). "Responsibilities of Health Care Professionals in Counseling and Educating Patients With Incurable Neurological Diseases Regarding "Stem Cell Tourism": Caveat Emptor". JAMA Neurology. 72 (11): 1342–1345. doi:10.1001/jamaneurol.2015.1891. PMID 26322563.
- ↑ Commissioner, Office of the (2020-09-09). "FDA Warns About Stem Cell Therapies". FDA.
- ↑ Commissioner, Office of the (2020-03-24). "FDA seeks permanent injunctions against two stem cell clinics". FDA. Retrieved 2021-02-09.
- ↑ "Communicating About Unproven Stem Cell Treatments to the Public". www.isscr.org.
- ↑ Tsou, Amy (February 10, 2015). "Ethical considerations when counseling patients about stem cell tourism". Continuum (Minneapolis, Minn.). 21 (1 Spinal Cord Disorders): 201–205. doi:10.1212/01.CON.0000461094.76563.be. PMID 25651226. S2CID 29862332.
- ↑ Du, Li; Rachul, Christen; Guo, Zhaochen; Caulfield, Timothy (January 10, 2016). "Gordie Howe's "Miraculous Treatment": Case Study of Twitter Users' Reactions to a Sport Celebrity's Stem Cell Treatment". JMIR Public Health and Surveillance. 2 (1): e8. doi: 10.2196/publichealth.5264 . PMC 4869214 . PMID 27227162.
- ↑ Shapiro, R. S. (2017). "Reconciling States' "Right to Try" Legislation and FDA's Expanded Access Program: Legal Issues". Therapeutic Innovation & Regulatory Science. 51 (2): 153–156. doi:10.1177/2168479016683217. PMID 30231723. S2CID 52301267.
- ↑ Speers, M. A. (2019). "Providing Patients with Critical or Life-Threatening Illnesses Access to Experimental Drug Therapy: A Guide to Clinical Trials and the US FDA Expanded Access Program". Pharmaceutical Medicine. 33 (2): 89–98. doi:10.1007/s40290-019-00274-3. PMID 31933253. S2CID 109920343.
- ↑ Kim, O. (2019). "A Response to Meyerson's Defence of the American Right to Try : Experimenting with hope". Journal of Bioethical Inquiry. 16 (3): 463–466. doi:10.1007/s11673-019-09918-0. PMID 31444643. S2CID 201285272.
- ↑ Simmons, Z. (2017). "Right-to-Try Investigational Therapies for Incurable Disorders". Continuum (Minneapolis, Minn.). 23 (5, Peripheral Nerve and Motor Neuron Disorders): 1451–1457. doi:10.1212/CON.0000000000000515. PMID 28968371. S2CID 43161837.