
The United States Food and Drug Administration is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, animal foods & feed and veterinary products.

Frank Edward Young was an American physician who served as Commissioner of Food and Drugs from 1984 to 1989 and later as a Deputy Assistant Secretary in the United States Department of Health and Human Services. In 2013 he joined Braeburn Pharmaceuticals as Executive Vice President, Clinical and Regulatory Affairs. In 2018, he became the Executive Vice President of Clinical and Regulatory Affairs at TissueTech Inc.
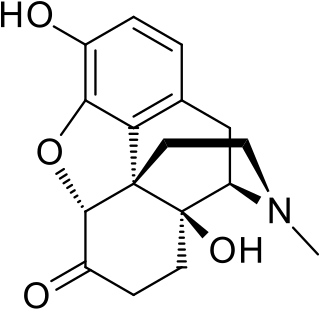
Oxymorphone is a highly potent opioid analgesic indicated for treatment of severe pain. Pain relief after injection begins after about 5–10 minutes, after oral administration it begins after about 30 minutes, and lasts about 3–4 hours for immediate-release tablets and 12 hours for extended-release tablets. The elimination half-life of oxymorphone is much faster intravenously, and as such, the drug is most commonly used orally. Like oxycodone, which metabolizes to oxymorphone, oxymorphone has a high potential to be abused.

The Center for Veterinary Medicine (CVM) is a branch of the U.S. Food and Drug Administration (FDA) that regulates the manufacture and distribution of food, food additives, and drugs that will be given to animals. These include animals from which human foods are derived, as well as food additives and drugs for pets or companion animals. CVM is responsible for regulating drugs, devices, and food additives given to, or used on, over one hundred million companion animals, plus millions of poultry, cattle, swine, and minor animal species. Minor animal species include animals other than cattle, swine, chickens, turkeys, horses, dogs, and cats.

Gardasil is an HPV vaccine for use in the prevention of certain strains of human papillomavirus (HPV). It was developed by Merck & Co. High-risk human papilloma virus (hr-HPV) genital infection is the most common sexually transmitted infection among women. The HPV strains that Gardasil protects against are sexually transmitted, specifically HPV types 6, 11, 16 and 18. HPV types 16 and 18 cause an estimated 70% of cervical cancers, and are responsible for most HPV-induced anal, vulvar, vaginal, and penile cancer cases. HPV types 6 and 11 cause an estimated 90% of genital warts cases. HPV type 16 is responsible for almost 90% of HPV-positive oropharyngeal cancers, and the prevalence is higher in males than females. Though Gardasil does not treat existing infection, vaccination is still recommended for HPV-positive individuals, as it may protect against one or more different strains of the disease.

Herbert Leonard Ley Jr. was an American physician and the 10th Commissioner and head of the U.S. Food and Drug Administration (FDA).

Stephen Michael Hahn is an American physician who served as the Commissioner of Food and Drugs from 2019 to 2021. Before becoming Commissioner, he was an oncologist serving as Chief Medical Executive of the MD Anderson Cancer Center.

The United States Commissioner of Food and Drugs is the head of the Food and Drug Administration (FDA), an agency of the United States Department of Health and Human Services. The commissioner is appointed by the president of the United States and must be confirmed by the Senate. The commissioner reports to the Secretary of Health and Human Services.

Margaret Ann "Peggy" Hamburg is an American physician and public health administrator, who is serving as the chair of the board of the American Association for the Advancement of Science (AAAS) and co-chair of the InterAcademy Partnership (IAP). She served as the 21st Commissioner of the U.S. Food and Drug Administration from May 2009 to April 2015.

Lester Mills Crawford is an American veterinarian and former Commissioner of the U.S. Food and Drug Administration who was appointed by President George W. Bush. He served from July 18, 2005 until resigning two months later in September 2005.

Boris Lushniak is a retired United States Public Health Service Commissioned Corps rear admiral who served as the acting Surgeon General of the United States, from July 17, 2013 to December 18, 2014. He previously served as the Deputy Surgeon General from 2010 to 2013 and from 2014 to 2015 when Vivek Murthy assumed office as Surgeon General. He retired from the Public Health Service on December 8, 2015 after over 27 years of service. On October 4, 2016 he was appointed dean of the University of Maryland, College Park School of Public Health, effective January 9, 2017.

Brett P. Giroir is an American pediatrician, former U.S. assistant secretary for health, four-star admiral in the U.S. Public Health Service Commissioned Corps and acting FDA commissioner. Dr. Giroir currently serves as CEO and a member of the Board of Directors for Altesa BioSciences, Inc, a clinical stage biopharmaceutical company focusing on developing new treatments for respiratory viruses and global viral threats. He is also a co-founder and independent director for Revelar Biotherapeutics, and an independent director for OncoNano Medicine. His non-profit activities include Board service on the Global Virus Network and Remote Area Medical (RAM), and he remains active in global humanitarian initiatives in Latin America and Sub-Saharan Africa. He previously served as the 16th assistant secretary for health during the Trump administration from February 15, 2018, to January 19, 2021. He concurrently served as the secretary's principal public health and science adviser, the senior adviser for the Health Resources and Services Administration, the Centers for Disease Control and Prevention, and the Substance Abuse and Mental Health Services Administration as well as the senior adviser to the secretary for Opioid Policy. From 2020 to 2021, he served additionally as the director of the U.S. coronavirus diagnostic testing, and as the U.S. representative on the World Health Organization Executive Board. As COVID-19 testing czar, he was part of the White House Coronavirus Task Force.

Luciana Borio is a Brazilian-American infectious disease physician and public health administrator. She is a vice president at In-Q-Tel. She previously served as director for Medical and Biodefense Preparedness at the National Security Council, acting chief scientist of the U.S. Food and Drug Administration (FDA), assistant commissioner for counterterrorism policy of the FDA, and director of FDA's Office of Counterterrorism and Emerging Threats. She is known for her work advancing clinical trials, the development of medical countermeasures for health emergencies, and the public health responses to Ebola and Zika outbreaks.

Robert McKinnon Califf is an American cardiologist who currently serves as the 25th Commissioner of the Food and Drug Administration.

Timothy R. Coté is an American doctor, expert of infectious and neoplastic disease, and a former Director of the Office of Orphan Products Development (OOPD) at the Food and Drug Administration (FDA).

Scott Gottlieb is an American physician and investor who served as the 23rd commissioner of the Food and Drug Administration (FDA) from May 2017 until April 2019. He is presently a senior fellow at the conservative think tank the American Enterprise Institute (AEI), a partner at the venture capital firm New Enterprise Associates (NEA), a member of the board of directors of drug maker Pfizer, Inc, a member of the board of directors of Illumina, Inc., a contributor to the cable financial news network CNBC, and a frequent guest on the CBS News program Face the Nation. An elected member of the National Academy of Medicine, Gottlieb is the author of The New York Times best selling book Uncontrolled Spread on the COVID-19 pandemic and the national security vulnerabilities that it revealed.

Janet Woodcock is an American physician and former Acting Commissioner of the U.S. Food and Drug Administration (FDA). She joined the FDA in 1986, and has held a number of senior leadership positions there, including terms as the Director of Center for Drug Evaluation and Research (CDER) from 1994 to 2004 and 2007 to 2021.

Food safety in the United States relates to the processing, packaging, and storage of food in a way that prevents food-borne illness within the United States. The beginning of regulation on food safety in the United States started in the early 1900s, when several outbreaks sparked the need for litigation managing food in the food industry. Over the next few decades, the United States created several government agencies in an effort to better understand contaminants in food and to regulate these impurities. Many laws regarding food safety in the United States have been created and amended since the beginning of the 1900s.

During his term as president of the United States (2017–2021), Donald Trump and his administration repeatedly politicized science by pressuring or overriding health and science agencies to change their reporting and recommendations so as to conform to his policies and public comments. This was particularly true with regard to the COVID-19 pandemic in the United States, but also included suppressing research on climate change and weakening or eliminating environmental regulations.


















