
Sir John Anthony Pople was a British theoretical chemist who was awarded the Nobel Prize in Chemistry with Walter Kohn in 1998 for his development of computational methods in quantum chemistry.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.

A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium CH+
3, methanium CH+
5 and vinyl C
2H+
3 cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered.

George Andrew Olah was a Hungarian-American chemist. His research involved the generation and reactivity of carbocations via superacids. For this research, Olah was awarded a Nobel Prize in Chemistry in 1994 "for his contribution to carbocation chemistry." He was also awarded the Priestley Medal, the highest honor granted by the American Chemical Society and F.A. Cotton Medal for Excellence in Chemical Research of the American Chemical Society in 1996.
Cyclohexa-1,3-diene (also known as Benzane) is an organic compound with the formula (C2H4)(CH)4. It is a colorless, flammable liquid. Its refractive index is 1.475 (20 °C, D). It is one of two isomers of cyclohexadiene, the other being 1,4-cyclohexadiene.
Frank Clifford Whitmore, nicknamed "Rocky", was a prominent chemist who submitted significant evidence for the existence of carbocation mechanisms in organic chemistry.
Clayton Heathcock is an organic chemist, professor of chemistry, and dean of the college of chemistry at the University of California, Berkeley. Heathcock is well known for his accomplishments in the synthesis of complex polycyclic natural products and for his contributions to the chemistry community. In 1995 he became a member of the National Academy of Sciences.

Dewar benzene (also spelled dewarbenzene) or bicyclo[2.2.0]hexa-2,5-diene is a bicyclic isomer of benzene with the molecular formula C6H6. The compound is named after James Dewar who included this structure in a list of possible C6H6 structures in 1869. However, he did not propose it as the structure of benzene, and in fact he supported the correct structure previously proposed by August Kekulé in 1865.
Reynold Clayton Fuson was an American chemist.
Fred Basolo was an American inorganic chemist. He received his Ph.D. at the University of Illinois at Urbana-Champaign in 1943, under Prof. John C. Bailar, Jr. Basolo spent his professional career at Northwestern University. He was a prolific contributor to the fields of coordination chemistry, organometallic, and bioinorganic chemistry, publishing over 400 papers. He supervised many Ph.D. students. With colleague Ralph Pearson, he co-authored the influential monograph "Mechanisms of Inorganic Reactions", which illuminated the importance of mechanisms involving coordination compounds. This work, which integrated concepts from ligand field theory and physical organic chemistry, signaled a shift from a highly descriptive nature of coordination chemistry to a more quantitative science.
Physical organic chemistry, a term coined by Louis Hammett in 1940, refers to a discipline of organic chemistry that focuses on the relationship between chemical structures and reactivity, in particular, applying experimental tools of physical chemistry to the study of organic molecules. Specific focal points of study include the rates of organic reactions, the relative chemical stabilities of the starting materials, reactive intermediates, transition states, and products of chemical reactions, and non-covalent aspects of solvation and molecular interactions that influence chemical reactivity. Such studies provide theoretical and practical frameworks to understand how changes in structure in solution or solid-state contexts impact reaction mechanism and rate for each organic reaction of interest.
Ernest Ludwig Eliel was an organic chemist born in Cologne, Germany. Among his awards were the Priestley Medal in 1996 and the NAS Award for Chemistry in Service to Society in 1997.

Peter John Stang is an American chemist and Distinguished Professor of chemistry at the University of Utah. He was the editor-in-chief of the Journal of the American Chemical Society from 2002 to 2020.

Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each been replaced by a methyl group. In 1929, Kathleen Lonsdale reported the crystal structure of hexamethylbenzene, demonstrating that the central ring is hexagonal and flat and thereby ending an ongoing debate about the physical parameters of the benzene system. This was a historically significant result, both for the field of X-ray crystallography and for understanding aromaticity.
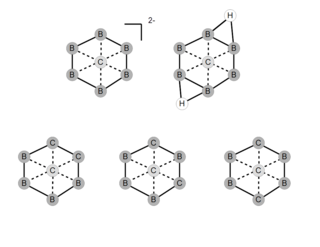
Planar hexacoordinate carbon in chemistry describes a molecular geometry featuring a planar arrangement of carbon with six surrounding atoms. No actual chemical compounds having this particular hexacoordinate configuration have been reported but quantum mechanical methods have demonstrated that these molecules are a possibility. Examples of molecules investigated with computational methods are the B6C dianion, the CN3Be3+ ion, the CO3Li3+ ion and the CN3Mg3+ ion. A simulated Be2C monolayer is reported to consist of quasi-planar hexacoordinate carbon atoms.
Peter Richard Schreiner is a German chemist who is a professor at Justus Liebig University Giessen. As of 2022, his h-index is 73.
Stefan Grimme, is a German physical chemist. He completed a Ph.D. thesis on photochemistry at Technical University of Braunschweig in 1991, and has been a professor at the Universität Bonn since 2011. Grimme is active in the field of computational chemistry and was elected a member of the Academy of Sciences Leopoldina in 2018.

Christopher J. Cramer is a research chemist and served as vice president for research at the University of Minnesota from 2018–2021. He presently serves as senior vice president and chief research officer for Underwriters Laboratories Inc.

Walter (W.) Dean Harman is an American chemist, academic, author and researcher. He is the William R. Kenan, Jr. Professor of Chemistry at the University of Virginia.










