
Steelmaking is the process of producing steel from iron ore and/or scrap. In steelmaking, impurities such as nitrogen, silicon, phosphorus, sulfur and excess carbon are removed from the sourced iron, and alloying elements such as manganese, nickel, chromium, carbon and vanadium are added to produce different grades of steel. Limiting dissolved gases such as nitrogen and oxygen and entrained impurities in the steel is also important to ensure the quality of the products cast from the liquid steel.
Coal gas is a flammable gaseous fuel made from coal and supplied to the user via a piped distribution system. It is produced when coal is heated strongly in the absence of air. Town gas is a more general term referring to manufactured gaseous fuels produced for sale to consumers and municipalities.
The Fischer–Tropsch process is a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen, known as syngas, into liquid hydrocarbons. These reactions occur in the presence of metal catalysts, typically at temperatures of 150–300 °C (302–572 °F) and pressures of one to several tens of atmospheres. The Fischer–Tropsch process is an important reaction in both coal liquefaction and gas to liquids technology for producing liquid hydrocarbons.
In industrial chemistry, coal gasification is the process of producing syngas—a mixture consisting primarily of carbon monoxide (CO), hydrogen, carbon dioxide, methane, and water vapour —from coal and water, air and/or oxygen.

Flue-gas desulfurization (FGD) is a set of technologies used to remove sulfur dioxide from exhaust flue gases of fossil-fuel power plants, and from the emissions of other sulfur oxide emitting processes such as waste incineration, petroleum refineries, cement and lime kilns.

A fossil fuel power station is a thermal power station which burns a fossil fuel, such as coal or natural gas, to produce electricity. Fossil fuel power stations have machinery to convert the heat energy of combustion into mechanical energy, which then operates an electrical generator. The prime mover may be a steam turbine, a gas turbine or, in small plants, a reciprocating gas engine. All plants use the energy extracted from the expansion of a hot gas, either steam or combustion gases. Although different energy conversion methods exist, all thermal power station conversion methods have their efficiency limited by the Carnot efficiency and therefore produce waste heat.

The Claus process is the most significant gas desulfurizing process, recovering elemental sulfur from gaseous hydrogen sulfide. First patented in 1883 by the chemist Carl Friedrich Claus, the Claus process has become the industry standard.
Amine gas treating, also known as amine scrubbing, gas sweetening and acid gas removal, refers to a group of processes that use aqueous solutions of various alkylamines (commonly referred to simply as amines) to remove hydrogen sulfide (H2S) and carbon dioxide (CO2) from gases. It is a common unit process used in refineries, and is also used in petrochemical plants, natural gas processing plants and other industries.

Flue gas is the gas exiting to the atmosphere via a flue, which is a pipe or channel for conveying exhaust gases from a fireplace, oven, furnace, boiler or steam generator. Quite often, the flue gas refers to the combustion exhaust gas produced at power plants. Its composition depends on what is being burned, but it will usually consist of mostly nitrogen derived from the combustion of air, carbon dioxide, and water vapor as well as excess oxygen. It further contains a small percentage of a number of pollutants, such as particulate matter, carbon monoxide, nitrogen oxides, and sulfur oxides.
Coal liquefaction is a process of converting coal into liquid hydrocarbons: liquid fuels and petrochemicals. This process is often known as "Coal to X" or "Carbon to X", where X can be many different hydrocarbon-based products. However, the most common process chain is "Coal to Liquid Fuels" (CTL).

Synthetic fuel or synfuel is a liquid fuel, or sometimes gaseous fuel, obtained from syngas, a mixture of carbon monoxide and hydrogen, in which the syngas was derived from gasification of solid feedstocks such as coal or biomass or by reforming of natural gas.

Coal pollution mitigation, sometimes called clean coal, is a series of systems and technologies that seek to mitigate the health and environmental impact of coal; in particular air pollution from coal-fired power stations, and from coal burnt by heavy industry.

Industrial gases are the gaseous materials that are manufactured for use in industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other gases and mixtures are also available in gas cylinders. The industry producing these gases is also known as industrial gas, which is seen as also encompassing the supply of equipment and technology to produce and use the gases. Their production is a part of the wider chemical Industry.

Hydrodesulfurization (HDS), also called hydrotreatment or hydrotreating, is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products, such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of removing the sulfur, and creating products such as ultra-low-sulfur diesel, is to reduce the sulfur dioxide emissions that result from using those fuels in automotive vehicles, aircraft, railroad locomotives, ships, gas or oil burning power plants, residential and industrial furnaces, and other forms of fuel combustion.
An integrated gasification combined cycle (IGCC) is a technology using a high pressure gasifier to turn coal and other carbon based fuels into pressurized gas—synthesis gas (syngas). It can then remove impurities from the syngas prior to the electricity generation cycle. Some of these pollutants, such as sulfur, can be turned into re-usable byproducts through the Claus process. This results in lower emissions of sulfur dioxide, particulates, mercury, and in some cases carbon dioxide. With additional process equipment, a water-gas shift reaction can increase gasification efficiency and reduce carbon monoxide emissions by converting it to carbon dioxide. The resulting carbon dioxide from the shift reaction can be separated, compressed, and stored through sequestration. Excess heat from the primary combustion and syngas fired generation is then passed to a steam cycle, similar to a combined cycle gas turbine. This process results in improved thermodynamic efficiency, compared to conventional pulverized coal combustion.
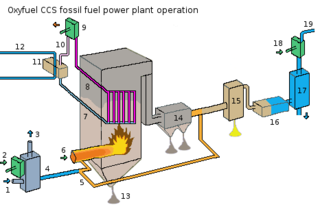
Oxy-fuel combustion is the process of burning a fuel using pure oxygen, or a mixture of oxygen and recirculated flue gas, instead of air. Since the nitrogen component of air is not heated, fuel consumption is reduced, and higher flame temperatures are possible. Historically, the primary use of oxy-fuel combustion has been in welding and cutting of metals, especially steel, since oxy-fuel allows for higher flame temperatures than can be achieved with an air-fuel flame. It has also received a lot of attention in recent decades as a potential carbon capture and storage technology.

Natural-gas processing is a range of industrial processes designed to purify raw natural gas by removing impurities, contaminants and higher molecular mass hydrocarbons to produce what is known as pipeline quality dry natural gas. Natural gas has to be processed in order to prepare it for final use and ensure that elimination of contaminants.

Natural gas has been used almost as long as crude oil in Canada, but its commercial development was not as rapid. This is because of special properties of this energy commodity: it is a gas, and it frequently contains impurities. The technical challenges involved to first process and then pipe it to market are therefore considerable. Furthermore, the costs of pipeline building make the whole enterprise capital intensive, requiring both money and engineering expertise, and large enough markets to make the business profitable.
The wet sulfuric acid process (WSA process) is a gas desulfurization process. After Danish company Haldor Topsoe introduced this technology in 1987, it has been recognized as a process for recovering sulfur from various process gases in the form of commercial quality sulfuric acid (H2SO4) with the simultaneous production of high-pressure steam. The WSA process can be applied in all industries where sulfur removal presents an issue.
The SNOX process is a process which removes sulfur dioxide, nitrogen oxides and particulates from flue gases. The sulfur is recovered as concentrated sulfuric acid and the nitrogen oxides are reduced to free nitrogen. The process is based on the well-known wet sulfuric acid process (WSA), a process for recovering sulfur from various process gasses in the form of commercial quality sulfuric acid (H2SO4).











