An explosive is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.

Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.

Nitrate is a polyatomic ion with the chemical formula NO−
3. Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insoluble nitrate is bismuth oxynitrate.
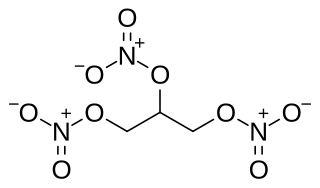
Nitroglycerin (NG), also known as trinitroglycerin (TNG), nitro, glyceryl trinitrate (GTN), or 1,2,3-trinitroxypropane, is a dense, colorless, oily, explosive liquid most commonly produced by nitrating glycerol with white fuming nitric acid under conditions appropriate to the formation of the nitric acid ester. Chemically, the substance is an organic nitrate compound rather than a nitro compound, but the traditional name is retained. Discovered in 1847 by Ascanio Sobrero, nitroglycerin has been used as an active ingredient in the manufacture of explosives, namely dynamite, and as such it is employed in the construction, demolition, and mining industries. It is combined with nitrocellulose to form double-based smokeless powder, which has been used as a propellant in artillery and firearms since the 1880s.

Nitric acid is the inorganic compound with the formula HNO3. It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%.

Trinitrotoluene, more commonly known as TNT, more specifically 2,4,6-trinitrotoluene, and by its preferred IUPAC name 2-methyl-1,3,5-trinitrobenzene, is a chemical compound with the formula C6H2(NO2)3CH3. TNT is occasionally used as a reagent in chemical synthesis, but it is best known as an explosive material with convenient handling properties. The explosive yield of TNT is considered to be the standard comparative convention of bombs and asteroid impacts. In chemistry, TNT is used to generate charge transfer salts.

Picric acid is an organic compound with the formula (O2N)3C6H2OH. Its IUPAC name is 2,4,6-trinitrophenol (TNP). The name "picric" comes from Greek: πικρός (pikros), meaning "bitter", due to its bitter taste. It is one of the most acidic phenols. Like other strongly nitrated organic compounds, picric acid is an explosive, which is its primary use. It has also been used as medicine (antiseptic, burn treatments) and as a dye.
Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer.

An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.

Lead(II) nitrate is an inorganic compound with the chemical formula Pb(NO3)2. It commonly occurs as a colourless crystal or white powder and, unlike most other lead(II) salts, is soluble in water.

In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid. The difference between the resulting molecular structures of nitro compounds and nitrates is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom, whereas in nitrate esters, the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom.

In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.

Silver fulminate (AgCNO) is the highly explosive silver salt of fulminic acid.

Resorcinol (or resorcin) is a phenolic compound. It is an organic compound with the formula C6H4(OH)2. It is one of three isomeric benzenediols, the 1,3-isomer (or meta-isomer). Resorcinol crystallizes from benzene as colorless needles that are readily soluble in water, alcohol, and ether, but insoluble in chloroform and carbon disulfide.

A contact explosive is a chemical substance that explodes violently when it is exposed to a relatively small amount of energy. Though different contact explosives have varying amounts of energy sensitivity, they are all much more sensitive relative to other kinds of explosives. Contact explosives are a part of a group of explosives called primary explosives, which are also very sensitive to stimuli but not to the degree of contact explosives. The extreme sensitivity of contact explosives is due to either chemical composition, bond type, or structure.
Nitrophenols are compounds of the formula HOC6H5−x(NO2)x. The conjugate bases are called nitrophenolates. Nitrophenols are more acidic than phenol itself.
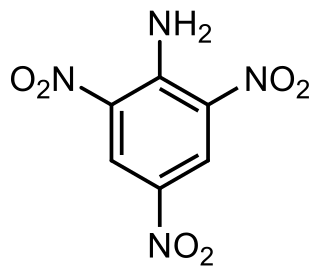
2,4,6-Trinitroaniline, C6H4N4O6, abbreviated as TNA and also known as picramide, a nitrated amine. Materials in this group range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups. The appearance of trinitroaniline varies from yellow to orange to red depending on its purity and concentration.

Potassium picrate, or potassium 2,4,6-trinitrophenolate, is an organic chemical, a picrate of potassium. It is a reddish yellow or green crystalline material. It is a primary explosive. Anhydrous potassium picrate forms orthorhombic crystals.

Trinitroanisole is a chemical compound that exists as pale yellow crystals with a melting point of 68 °C. It is highly toxic. It is an explosive with a detonation velocity of 7200 meters per second. The compound's primary hazard is a blast of an instantaneous explosion, not flying projectiles or fragments.

In organic chemistry, a nitrate ester is an organic functional group with the formula R−ONO2, where R stands for any organyl group. They are the esters of nitric acid and alcohols. A well-known example is nitroglycerin, which is not a nitro compound, despite its name.