
In organic chemistry, a ketone is a functional group with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.
In organic chemistry, the Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide (DMSO) and an organic base, such as triethylamine. It is one of the many oxidation reactions commonly referred to as 'activated DMSO' oxidations. The reaction is known for its mild character and wide tolerance of functional groups.

Pyridinium chlorochromate (PCC) is a yellow-orange salt with the formula [C5H5NH]+[CrO3Cl]−. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity. PCC offers the advantage of the selective oxidation of alcohols to aldehydes or ketones, whereas many other reagents are less selective.
The Kornblum oxidation, named after Nathan Kornblum, is an organic oxidation reaction that converts alkyl halides and tosylates into carbonyl compounds.

Dess–Martin periodinane (DMP) is a chemical reagent used in the Dess–Martin oxidation, oxidizing primary alcohols to aldehydes and secondary alcohols to ketones. This periodinane has several advantages over chromium- and DMSO-based oxidants that include milder conditions, shorter reaction times, higher yields, simplified workups, high chemoselectivity, tolerance of sensitive functional groups, and a long shelf life. However, use on an industrial scale is made difficult by its cost and its potentially explosive nature. It is named after the American chemists Daniel Benjamin Dess and James Cullen Martin who developed the reagent in 1983. It is based on IBX, but due to the acetate groups attached to the central iodine atom, DMP is much more reactive than IBX and is much more soluble in organic solvents.

Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen, respectively.
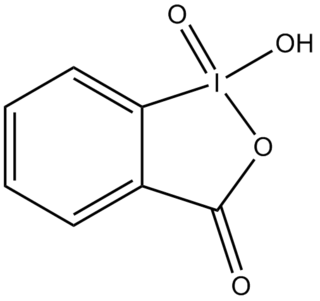
2-Iodoxybenzoic acid (IBX) is an organic compound used in organic synthesis as an oxidizing agent. This periodinane is especially suited to oxidize alcohols to aldehydes. IBX is prepared from 2-iodobenzoic acid, potassium bromate, and sulfuric acid. Frigerio and co-workers have also demonstrated, in 1999 that potassium bromate may be replaced by commercially available Oxone. One of the main drawbacks of IBX is its limited solubility; IBX is insoluble in many common organic solvents. In the past, it was believed that IBX was shock sensitive, but it was later proposed that samples of IBX were shock sensitive due to the residual potassium bromate left from its preparation. Commercial IBX is stabilized by carboxylic acids such as benzoic acid and isophthalic acid.
The Pfitzner–Moffatt oxidation, sometimes referred to as simply the Moffatt oxidation, is a chemical reaction for the oxidation of primary and secondary alcohols to aldehydes and ketones, respectively. The oxidant is a combination of dimethyl sulfoxide (DMSO) and dicyclohexylcarbodiimide (DCC). The reaction was first reported by J. Moffatt and his student K. Pfitzner in 1963.
The Corey–Kim oxidation is an oxidation reaction used to synthesise aldehydes and ketones from primary and secondary alcohols. It is named for American chemist and Nobel Laureate Elias James Corey and Korean-American chemist Choung Un Kim.
Oppenauer oxidation, named after Rupert Viktor Oppenauer, is a gentle method for selectively oxidizing secondary alcohols to ketones.
In organic chemistry, the Ei mechanism, also known as a thermal syn elimination or a pericyclic syn elimination, is a special type of elimination reaction in which two vicinal (adjacent) substituents on an alkane framework leave simultaneously via a cyclic transition state to form an alkene in a syn elimination. This type of elimination is unique because it is thermally activated and does not require additional reagents, unlike regular eliminations, which require an acid or base, or would in many cases involve charged intermediates. This reaction mechanism is often found in pyrolysis.

The oxidation of alcohols to carbonyls is an important oxidation reaction in organic chemistry. This only occurs with primary and secondary alcohols.

Methanesulfonic anhydride (Ms2O) is the acid anhydride of methanesulfonic acid. Like methanesulfonyl chloride (MsCl), it may be used to generate mesylates (methanesulfonyl esters).
The Parikh–Doering oxidation is an oxidation reaction that transforms primary and secondary alcohols into aldehydes and ketones, respectively. The procedure uses dimethyl sulfoxide (DMSO) as the oxidant and the solvent, activated by the sulfur trioxide pyridine complex (SO3•C5H5N) in the presence of triethylamine or diisopropylethylamine as base. Dichloromethane is frequently used as a cosolvent for the reaction.
Alcohol oxidation is a class of organic reactions in which the alcohol functional group is converted into another functional group in which carbon carries a higher oxidation state.
Oxidation with chromium(VI) complexes involves the conversion of alcohols to carbonyl compounds or more highly oxidized products through the action of molecular chromium(VI) oxides and salts. The principal reagents are Collins reagent, PDC, and PCC. These reagents represent improvements over inorganic chromium(VI) reagents such as Jones reagent.

Oxoammonium-catalyzed oxidation reactions involve the conversion of organic substrates to more highly oxidized materials through the action of an N-oxoammonium species. Nitroxides may also be used in catalytic amounts in the presence of a stoichiometric amount of a terminal oxidant. Nitroxide radical species used are either 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) or derivatives thereof.
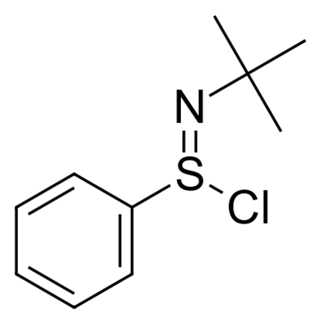
N-tert-Butylbenzenesulfinimidoyl chloride is a useful oxidant for organic synthesis reactions. It is a good electrophile, and the sulfimide S=N bond can be attacked by nucleophiles, such as alkoxides, enolates, and amide ions. The nitrogen atom in the resulting intermediate is basic, and can abstract an α-hydrogen to create a new double bond.
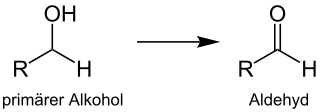
The Albright–Goldman oxidation is a name reaction of organic chemistry, first described by the American chemists J. Donald Albright and Leon Goldman in 1965. The reaction is particularly suitable for the synthesis of aldehydes from primary alcohols. Analogously, secondary alcohols can be oxidized to form ketones. Dimethyl sulfoxide/acetic anhydride serves as oxidizing agent.











