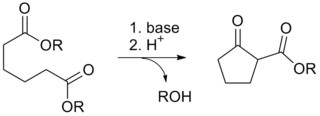In organic chemistry, amines (, UK also ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; see Category:Amines for a list of amines. Inorganic derivatives of ammonia are also called amines, such as monochloramine (NClH2).

Ethers are a class of organic compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R–O–R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether" (CH3–CH2–O–CH2–CH3). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

In chemistry, a ketone is a functional group with the structure R2C=O, where R can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond). The simplest ketone is acetone (R = R' = methyl), with the formula CH3C(O)CH3. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone.

A ketene is an organic compound of the form R′R″C=C=O, where R and R' are two arbitrary monovalent chemical groups. The name may also refer to the specific compound ethenone H
2C=C=O, the simplest ketene.

In chemistry thioesters are compounds with the functional group R–S–CO–R'. They are analogous to carboxylate esters with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester. They are the product of esterification between a carboxylic acid and a thiol. In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.

A dicarbonyl is a molecule containing two carbonyl (C=O) groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical or unsymmetrical.
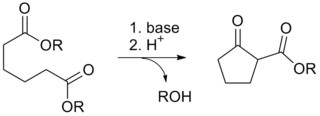
The Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-keto esters. It is named after the German chemist Walter Dieckmann (1869–1925). The equivalent intermolecular reaction is the Claisen condensation.
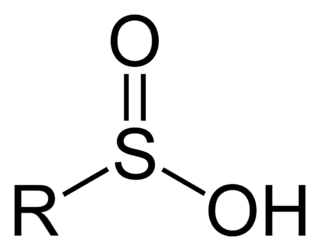
Sulfinic acids are oxoacids of sulfur with the structure RSO(OH). In these organosulfur compounds, sulfur is pyramidal..

A sulfenic acid is an organosulfur compound and oxoacid with the general formula RSOH. It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids and sulfonic acids, RSO2H and RSO3H, respectively. The base member of the sulfenic acid series with R = H is hydrogen thioperoxide.

Thioacetic acid is an organosulfur compound with the molecular formula CH3COSH. It is a yellow liquid with a strong thiol-like odor. It is used in organic synthesis for the introduction of thiol groups in molecules.

Hypotaurine is a sulfinic acid that is an intermediate in the biosynthesis of taurine. Like taurine, it also acts as an endogenous neurotransmitter via action on the glycine receptors. It is an osmolyte with antioxidant properties.
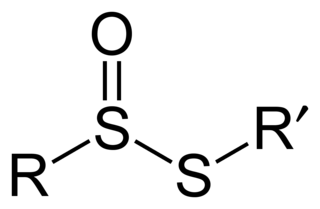
In organosulfur chemistry, thiosulfinate is a functional group consisting of the linkage R-S(O)-S-R (R are organic substituents). Thiolsulfinates are also named as alkanethiosulfinic (or arenethiosulfinic) acid esters. They are the first member of a family of compounds containing an oxidized disulfide bond. Other members of this family include thiosulfonates (R-SO2-S-R), α-disulfoxides (R-S(O)-S(O)-R), sulfinyl sulfones (R-S(O)-SO2-R), and α-disulfones (R-SO2-SO2-R), all of which are known. The thiosulfinate group can occur in cyclic as well as acyclic structures.
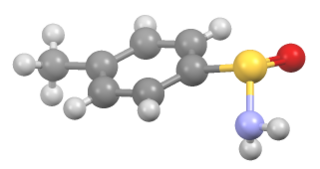
Sulfinamide is a functional group in organosulfur chemistry with the structural formula RS(O)NR'2. This functionality is composed of a sulfur-carbon (S-C) and sulfur-nitrogen (S-N) single bonds, as well as a sulfur-oxygen double bond (S=O), resulting in a tetravalent sulfur centre. As a non-bonding electron pair is also present on the sulfur, these compounds are also chiral. They are sometimes referred to as S-chiral sulfinamides. Sulfinamides are amides of sulfinic acid.
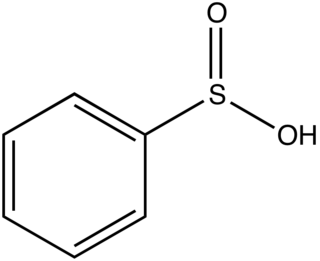
Phenylsulfinic acid is an organosulfur compound with the formula C6H5SO2H. It is a colorless or white crystalline solid that is usually stored in the form of its sodium salt. In aqueous solution it is strongly acidic and is easily oxidized in air. Phenylsulfinic acid and its esters are chiral.

Sulfinyl halide groups occur when a sulfinyl functional group is singly bonded to a halogen atom. They have the general formula R-S(O)-X, where X is a halogen, and are intermediate in oxidation level between sulfenyl halides, R-S-X, and sulfonyl halides, R-SO2-X. The best known examples are sulfinyl chlorides, thermolabile, moisture-sensitive compounds, which are useful intermediates for preparation of other sufinyl derivatives such as sulfinamides, sulfinates, sulfoxides and thiosulfinates. Unlike the sulfur atom in sulfonyl halides and sulfenyl halides, the sulfur atom in sulfinyl halides is chiral, as shown for methanesulfinyl chloride.

Thiosulfurous acid (HS−S(=O)−OH) is a hypothetical compound with the formula S2(OH)2. Attempted synthesis leads to polymers. It is a low oxidation state (+1) sulfur acid. It is the equivalent acid for disulfur monoxide. Salts derived from thiosulfurous acid, which are also unknown, are named "thiosulfites" or "sulfurothioites". The ion is S=SO2−
2.

In chemistry, the sulfonamide functional group (also spelled sulphonamide) is -S(=O)2-NH2, a sulfonyl group connected to an amine group. Relatively speaking this group is unreactive. The amine center is no longer basic. The S-N bond is cleaved only with difficulty. Because of the rigidity of the functional group, sulfonamides are typically crystalline. For this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group.

Thiobenzoic acid is an organosulfur compound with molecular formula C6H5COSH. It is the parent of aryl thiocarboxylic acids. It is a pale yellow liquid that freezes just below room temperature.

Sulfoxylic acid (H2SO2) (also known as hyposulfurous acid or sulfur dihydroxide) is an unstable oxoacid of sulfur in an intermediate oxidation state between hydrogen sulfide and dithionous acid. It consists of two hydroxy groups attached to a sulfur atom. Sulfoxylic acid contains sulfur in an oxidation state of +2. Sulfur monoxide (SO) can be considered as a theoretical anhydride for sulfoxylic acid, but it is not actually known to react with water.

α,β-Unsaturated carbonyl compounds refers to organic compounds with the general structure (O=CR)−Cα=Cβ-R. Examples would be enones and enals. In these compounds the carbonyl group is conjugated with an alkene. Unlike the case for carbonyls without a flanking alkene group, α,β-unsaturated carbonyl compounds are susceptible to attack by nucleophiles at the β carbon. This pattern of reactivity is called vinylogous. Examples of unsaturated carbonyls are acrolein (propenal), mesityl oxide, acrylic acid, and maleic acid. Unsaturated carbonyls can be prepared in the laboratory in an aldol reaction and in the Perkin reaction.