Related Research Articles
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.

High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can originate from food, chemicals, pharmaceuticals, biological, environmental and agriculture, etc, which have been dissolved into liquid solutions.

Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.
A supercritical fluid (SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist, but below the pressure required to compress it into a solid. It can effuse through porous solids like a gas, overcoming the mass transfer limitations that slow liquid transport through such materials. SCF are much superior to gases in their ability to dissolve materials like liquids or solids. Also, near the critical point, small changes in pressure or temperature result in large changes in density, allowing many properties of a supercritical fluid to be "fine-tuned".

Column chromatography in chemistry is a chromatography method used to isolate a single chemical compound from a mixture. Chromatography is able to separate substances based on differential adsorption of compounds to the adsorbent; compounds move through the column at different rates, allowing them to be separated into fractions. The technique is widely applicable, as many different adsorbents can be used with a wide range of solvents. The technique can be used on scales from micrograms up to kilograms. The main advantage of column chromatography is the relatively low cost and disposability of the stationary phase used in the process. The latter prevents cross-contamination and stationary phase degradation due to recycling. Column chromatography can be done using gravity to move the solvent, or using compressed gas to push the solvent through the column.

Liquid chromatography–mass spectrometry (LC–MS) is an analytical chemistry technique that combines the physical separation capabilities of liquid chromatography with the mass analysis capabilities of mass spectrometry (MS). Coupled chromatography – MS systems are popular in chemical analysis because the individual capabilities of each technique are enhanced synergistically. While liquid chromatography separates mixtures with multiple components, mass spectrometry provides spectral information that may help to identify each separated component. MS is not only sensitive, but provides selective detection, relieving the need for complete chromatographic separation. LC–MS is also appropriate for metabolomics because of its good coverage of a wide range of chemicals. This tandem technique can be used to analyze biochemical, organic, and inorganic compounds commonly found in complex samples of environmental and biological origin. Therefore, LC–MS may be applied in a wide range of sectors including biotechnology, environment monitoring, food processing, and pharmaceutical, agrochemical, and cosmetic industries. Since the early 2000s, LC–MS has also begun to be used in clinical applications.

Solid-phase extraction (SPE) is a solid-liquid extractive technique, by which compounds that are dissolved or suspended in a liquid mixture are separated, isolated or purified, from other compounds in this mixture, according to their physical and chemical properties. Analytical laboratories use solid phase extraction to concentrate and purify samples for analysis. Solid phase extraction can be used to isolate analytes of interest from a wide variety of matrices, including urine, blood, water, beverages, soil, and animal tissue.
Chiral column chromatography is a variant of column chromatography that is employed for the separation of chiral compounds, i.e. enantiomers, in mixtures such as racemates or related compounds. The chiral stationary phase (CSP) is made of a support, usually silica based, on which a chiral reagent or a macromolecule with numerous chiral centers is bonded or immobilized.
Reversed-phase liquid chromatography (RP-LC) is a mode of liquid chromatography in which non-polar stationary phase and polar mobile phases are used for the separation of organic compounds. The vast majority of separations and analyses using high-performance liquid chromatography (HPLC) in recent years are done using the reversed phase mode. In the reversed phase mode, the sample components are retained in the system the more hydrophobic they are.
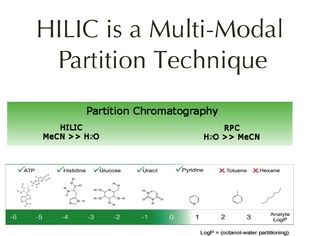
Hydrophilic interaction chromatography is a variant of normal phase liquid chromatography that partly overlaps with other chromatographic applications such as ion chromatography and reversed phase liquid chromatography. HILIC uses hydrophilic stationary phases with reversed-phase type eluents. The name was suggested by Andrew Alpert in his 1990 paper on the subject. He described the chromatographic mechanism for it as liquid-liquid partition chromatography where analytes elute in order of increasing polarity, a conclusion supported by a review and re-evaluation of published data.
Micellar liquid chromatography (MLC) is a form of reversed phase liquid chromatography that uses an aqueous micellar solutions as the mobile phase.
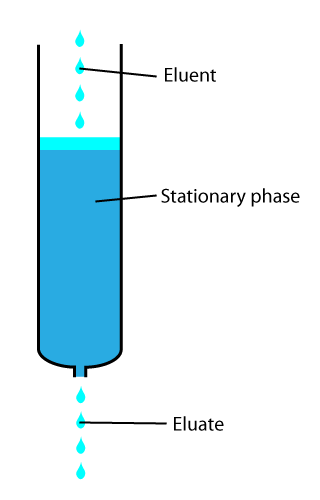
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions.

Superheated water is liquid water under pressure at temperatures between the usual boiling point, 100 °C (212 °F) and the critical temperature, 374 °C (705 °F). It is also known as "subcritical water" or "pressurized hot water". Superheated water is stable because of overpressure that raises the boiling point, or by heating it in a sealed vessel with a headspace, where the liquid water is in equilibrium with vapour at the saturated vapor pressure. This is distinct from the use of the term superheating to refer to water at atmospheric pressure above its normal boiling point, which has not boiled due to a lack of nucleation sites.

Two-dimensional chromatography is a type of chromatographic technique in which the injected sample is separated by passing through two different separation stages. Two different chromatographic columns are connected in sequence, and the effluent from the first system is transferred onto the second column. Typically the second column has a different separation mechanism, so that bands that are poorly resolved from the first column may be completely separated in the second column. Alternately, the two columns might run at different temperatures. During the second stage of separation the rate at which the separation occurs must be faster than the first stage, since there is still only a single detector. The plane surface is amenable to sequential development in two directions using two different solvents.

Countercurrent chromatography is a form of liquid–liquid chromatography that uses a liquid stationary phase that is held in place by inertia of the molecules composing the stationary phase accelerating toward the center of a centrifuge due to centripetal force and is used to separate, identify, and quantify the chemical components of a mixture. In its broadest sense, countercurrent chromatography encompasses a collection of related liquid chromatography techniques that employ two immiscible liquid phases without a solid support. The two liquid phases come in contact with each other as at least one phase is pumped through a column, a hollow tube or a series of chambers connected with channels, which contains both phases. The resulting dynamic mixing and settling action allows the components to be separated by their respective solubilities in the two phases. A wide variety of two-phase solvent systems consisting of at least two immiscible liquids may be employed to provide the proper selectivity for the desired separation.
Atmospheric pressure laser ionization is an atmospheric pressure ionization method for mass spectrometry (MS). Laser light in the UV range is used to ionize molecules in a resonance-enhanced multiphoton ionization (REMPI) process. It is a selective and sensitive ionization method for aromatic and polyaromatic compounds. Atmospheric photoionization is the latest in development of atmospheric ionization methods.

In chemical analysis, capillary electrochromatography (CEC) is a chromatographic technique in which the mobile phase is driven through the chromatographic bed by electro-osmosis. Capillary electrochromatography is a combination of two analytical techniques, high-performance liquid chromatography and capillary electrophoresis. Capillary electrophoresis aims to separate analytes on the basis of their mass-to-charge ratio by passing a high voltage across ends of a capillary tube, which is filled with the analyte. High-performance liquid chromatography separates analytes by passing them, under high pressure, through a column filled with stationary phase. The interactions between the analytes and the stationary phase and mobile phase lead to the separation of the analytes. In capillary electrochromatography capillaries, packed with HPLC stationary phase, are subjected to a high voltage. Separation is achieved by electrophoretic migration of solutes and differential partitioning.
Thermoresponsive polymers can be used as stationary phase in liquid chromatography. Here, the polarity of the stationary phase can be varied by temperature changes, altering the power of separation without changing the column or solvent composition. Thermally related benefits of gas chromatography can now be applied to classes of compounds that are restricted to liquid chromatography due to their thermolability. In place of solvent gradient elution, thermoresponsive polymers allow the use of temperature gradients under purely aqueous isocratic conditions. The versatility of the system is controlled not only through changing temperature, but through the addition of modifying moieties that allow for a choice of enhanced hydrophobic interaction, or by introducing the prospect of electrostatic interaction. These developments have already introduced major improvements to the fields of hydrophobic interaction chromatography, size exclusion chromatography, ion exchange chromatography, and affinity chromatography separations as well as pseudo-solid phase extractions.
An evaporative light scattering detector (ELSD) is a destructive chromatography detector, used in conjunction with high-performance liquid chromatography (HPLC), ultra high-performance liquid chromatography (UHPLC), purification liquid chromatography such as flash or preparative chromatography, countercurrent or centrifugal partition chromatography and supercritical fluid chromatography (SFC). It is commonly used for analysis of compounds that do not absorb UV-VIS radiation significantly, such as sugars, antiviral drugs, antibiotics, fatty acids, lipids, oils, phospholipids, polymers, surfactants, terpenoids and triglycerides.
Chiral analysis refers to the quantification of component enantiomers of racemic drug substances or pharmaceutical compounds. Other synonyms commonly used include enantiomer analysis, enantiomeric analysis, and enantioselective analysis. Chiral analysis includes all analytical procedures focused on the characterization of the properties of chiral drugs. Chiral analysis is usually performed with chiral separation methods where the enantiomers are separated on an analytical scale and simultaneously assayed for each enantiomer.
References
- ↑ "3.3: Basic Principles of Supercritical Fluid Chromatography and Supercrtical Fluid Extraction". Chemistry LibreTexts. July 13, 2016.
- ↑ Taylor, Larry T. (2010). "Supercritical Fluid Chromatography". Analytical Chemistry. 82 (12): 4925–4935. doi:10.1021/ac101194x. ISSN 0003-2700. PMID 20465290.
- ↑ Taylor, Larry T. (2009). "Supercritical fluid chromatography for the 21st century". The Journal of Supercritical Fluids. 47 (3): 566–573. doi:10.1016/j.supflu.2008.09.012. ISSN 0896-8446.
- ↑
- ↑ Giddings, John Calvin (1968). "High Pressure Gas Chromatography of Nonvolatile Species: Compressed gas is used to cause migration of intractable solutes". Science. 162 (3849): 67–73. doi:10.1126/science.162.3849.67. PMID 5675186.
- ↑ Si-Hung, Le; Bamba, Takeshi (2022-04-01). "Current state and future perspectives of supercritical fluid chromatography". TrAC Trends in Analytical Chemistry. 149: 116550. doi:10.1016/j.trac.2022.116550. ISSN 0165-9936. S2CID 246461641.
- ↑ White, Craig; Burnett, John (2005). "Integration of supercritical fluid chromatography into drug discovery as a routine support tool". Journal of Chromatography A. 1074 (1–2): 175–185. doi:10.1016/j.chroma.2005.02.087. ISSN 0021-9673. PMID 15941053.
- ↑ Bower, Nathan W. (August 1992). "Principles of Instrumental Analysis. 4th edition (Skoog, D. A.; Leary, J. J.)". Journal of Chemical Education. 69 (8): A224. Bibcode:1992JChEd..69Q.224B. doi: 10.1021/ed069pA224.1 . ISSN 0021-9584.
- ↑ Lester Dolak (October 2004), "Carbon Dioxide Chromatography: The role of SFC in pharmaceutical discovery" (PDF), Today's Chemist at Work: 47–48