Molecular diffusion, often simply called diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of the particles. Diffusion explains the net flux of molecules from a region of higher concentration to one of lower concentration. Once the concentrations are equal the molecules continue to move, but since there is no concentration gradient the process of molecular diffusion has ceased and is instead governed by the process of self-diffusion, originating from the random motion of the molecules. The result of diffusion is a gradual mixing of material such that the distribution of molecules is uniform. Since the molecules are still in motion, but an equilibrium has been established, the result of molecular diffusion is called a "dynamic equilibrium". In a phase with uniform temperature, absent external net forces acting on the particles, the diffusion process will eventually result in complete mixing.

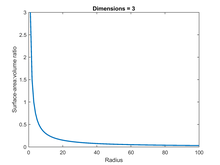
The surface area of a solid object is a measure of the total area that the surface of the object occupies. The mathematical definition of surface area in the presence of curved surfaces is considerably more involved than the definition of arc length of one-dimensional curves, or of the surface area for polyhedra, for which the surface area is the sum of the areas of its faces. Smooth surfaces, such as a sphere, are assigned surface area using their representation as parametric surfaces. This definition of surface area is based on methods of infinitesimal calculus and involves partial derivatives and double integration.
In continuum mechanics, the Péclet number is a class of dimensionless numbers relevant in the study of transport phenomena in a continuum. It is defined to be the ratio of the rate of advection of a physical quantity by the flow to the rate of diffusion of the same quantity driven by an appropriate gradient. In the context of species or mass transfer, the Péclet number is the product of the Reynolds number and the Schmidt number. In the context of the thermal fluids, the thermal Péclet number is equivalent to the product of the Reynolds number and the Prandtl number.
In thermal fluid dynamics, the Nusselt number is the ratio of convective to conductive heat transfer at a boundary in a fluid. Convection includes both advection and diffusion (conduction). The conductive component is measured under the same conditions as the convective but for a hypothetically motionless fluid. It is a dimensionless number, closely related to the fluid's Rayleigh number.
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by , , or and is measured in W·m−1·K−1.
In fluid mechanics, the Rayleigh number (Ra, after Lord Rayleigh) for a fluid is a dimensionless number associated with buoyancy-driven flow, also known as free (or natural) convection. It characterises the fluid's flow regime: a value in a certain lower range denotes laminar flow; a value in a higher range, turbulent flow. Below a certain critical value, there is no fluid motion and heat transfer is by conduction rather than convection. For most engineering purposes, the Rayleigh number is large, somewhere around 106 to 108.
Conduction is the process by which heat is transferred from the hotter end to the colder end of an object. The ability of the object to conduct heat is known as its thermal conductivity, and is denoted k.

Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid is dissolved by or permeates a liquid or solid. While adsorption does often precede absorption, which involves the transfer of the absorbate into the volume of the absorbent material, alternatively, adsorption is distinctly a surface phenomenon, wherein the adsorbate does not penetrate through the material surface and into the bulk of the adsorbent. The term sorption encompasses both adsorption and absorption, and desorption is the reverse of sorption.

Gas exchange is the physical process by which gases move passively by diffusion across a surface. For example, this surface might be the air/water interface of a water body, the surface of a gas bubble in a liquid, a gas-permeable membrane, or a biological membrane that forms the boundary between an organism and its extracellular environment.

Kleiber's law, named after Max Kleiber for his biology work in the early 1930s, is the observation that, for the vast majority of animals, an animal's metabolic rate scales to the 3⁄4 power of the animal's mass. More recently, Kleiber's law has also been shown to apply in plants, suggesting that Kleiber's observation is much more general. Symbolically: if B is the animal's metabolic rate, and M is the animal's mass, then Kleiber's law states that B~M3/4. Thus, over the same time span, a cat having a mass 100 times that of a mouse will consume only about 32 times the energy the mouse uses.

Allometry is the study of the relationship of body size to shape, anatomy, physiology and finally behaviour, first outlined by Otto Snell in 1892, by D'Arcy Thompson in 1917 in On Growth and Form and by Julian Huxley in 1932.

The square–cube law is a mathematical principle, applied in a variety of scientific fields, which describes the relationship between the volume and the surface area as a shape's size increases or decreases. It was first described in 1638 by Galileo Galilei in his Two New Sciences as the "...ratio of two volumes is greater than the ratio of their surfaces".
Osmotrophy is a feeding mechanism involving the movement of dissolved organic compounds by osmosis for nutrition. Organisms that use osmotrophy are called osmotrophs. Some mixotrophic microorganisms use osmotrophy to derive some of their energy. Osmotrophy is used by many diverse organisms. Organisms that use osmotrophy include bacteria, many species of protists and most fungi. Some macroscopic animals like molluscs, sponges, corals, brachiopods and echinoderms may use osmotrophic feeding as a supplemental food source.
In fluid dynamics, the Schmidt number of a fluid is a dimensionless number defined as the ratio of momentum diffusivity and mass diffusivity, and it is used to characterize fluid flows in which there are simultaneous momentum and mass diffusion convection processes. It was named after German engineer Ernst Heinrich Wilhelm Schmidt (1892–1975).

In electromagnetism, current density is the amount of charge per unit time that flows through a unit area of a chosen cross section. The current density vector is defined as a vector whose magnitude is the electric current per cross-sectional area at a given point in space, its direction being that of the motion of the positive charges at this point. In SI base units, the electric current density is measured in amperes per square metre.

Diffusion is the net movement of anything generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, as in spinodal decomposition. Diffusion is a stochastic process due to the inherent randomness of the diffusing entity and can be used to model many real-life stochastic scenarios. Therefore, diffusion and the corresponding mathematical models are used in several fields beyond physics, such as statistics, probability theory, information theory, neural networks, finance, and marketing.
Nuclear magnetic resonance (NMR) in porous materials covers the application of using NMR as a tool to study the structure of porous media and various processes occurring in them. This technique allows the determination of characteristics such as the porosity and pore size distribution, the permeability, the water saturation, the wettability, etc.
In engineering, physics, and chemistry, the study of transport phenomena concerns the exchange of mass, energy, charge, momentum and angular momentum between observed and studied systems. While it draws from fields as diverse as continuum mechanics and thermodynamics, it places a heavy emphasis on the commonalities between the topics covered. Mass, momentum, and heat transport all share a very similar mathematical framework, and the parallels between them are exploited in the study of transport phenomena to draw deep mathematical connections that often provide very useful tools in the analysis of one field that are directly derived from the others.
Microvasculature comprises the microvessels – venules and capillaries of the microcirculation, with a maximum average diameter of 0.3 millimeters. As the vessels decrease in size, they increase their surface-area-to-volume ratio. This allows surface properties to play a significant role in the function of the vessel.
In mathematical heat conduction, the Green's function number is used to uniquely categorize certain fundamental solutions of the heat equation to make existing solutions easier to identify, store, and retrieve.