Computational chemistry is a branch of chemistry that uses computer simulation to assist in solving chemical problems. It uses methods of theoretical chemistry, incorporated into computer programs, to calculate the structures and properties of molecules, groups of molecules, and solids. It is essential because, apart from relatively recent results concerning the hydrogen molecular ion, the quantum many-body problem cannot be solved analytically, much less in closed form. While computational results normally complement the information obtained by chemical experiments, it can in some cases predict hitherto unobserved chemical phenomena. It is widely used in the design of new drugs and materials.

Auger electron spectroscopy is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. It is a form of electron spectroscopy that relies on the Auger effect, based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in X-ray spectroscopy data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy, gas-phase chemistry, and throughout the microelectronics industry.

Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid–gas interfaces. It includes the fields of surface chemistry and surface physics. Some related practical applications are classed as surface engineering. The science encompasses concepts such as heterogeneous catalysis, semiconductor device fabrication, fuel cells, self-assembled monolayers, and adhesives. Surface science is closely related to interface and colloid science. Interfacial chemistry and physics are common subjects for both. The methods are different. In addition, interface and colloid science studies macroscopic phenomena that occur in heterogeneous systems due to peculiarities of interfaces.

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique based on the photoelectric effect that can identify the elements that exist within a material or are covering its surface, as well as their chemical state, and the overall electronic structure and density of the electronic states in the material. XPS is a powerful measurement technique because it not only shows what elements are present, but also what other elements they are bonded to. The technique can be used in line profiling of the elemental composition across the surface, or in depth profiling when paired with ion-beam etching. It is often applied to study chemical processes in the materials in their as-received state or after cleavage, scraping, exposure to heat, reactive gasses or solutions, ultraviolet light, or during ion implantation.
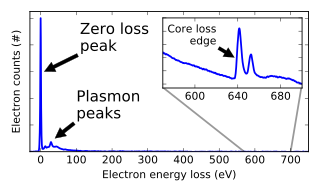
In electron energy loss spectroscopy (EELS) a material is exposed to a beam of electrons with a known, narrow range of kinetic energies. Some of the electrons will undergo inelastic scattering, which means that they lose energy and have their paths slightly and randomly deflected. The amount of energy loss can be measured via an electron spectrometer and interpreted in terms of what caused the energy loss. Inelastic interactions include phonon excitations, inter- and intra-band transitions, plasmon excitations, inner shell ionizations, and Cherenkov radiation. The inner-shell ionizations are particularly useful for detecting the elemental components of a material. For example, one might find that a larger-than-expected number of electrons comes through the material with 285 eV less energy than they had when they entered the material. This is approximately the amount of energy needed to remove an inner-shell electron from a carbon atom, which can be taken as evidence that there is a significant amount of carbon present in the sample. With some care, and looking at a wide range of energy losses, one can determine the types of atoms, and the numbers of atoms of each type, being struck by the beam. The scattering angle can also be measured, giving information about the dispersion relation of whatever material excitation caused the inelastic scattering.
Scattering is a term used in physics to describe a wide range of physical processes where moving particles or radiation of some form, such as light or sound, are forced to deviate from a straight trajectory by localized non-uniformities in the medium through which they pass. In conventional use, this also includes deviation of reflected radiation from the angle predicted by the law of reflection. Reflections of radiation that undergo scattering are often called diffuse reflections and unscattered reflections are called specular (mirror-like) reflections. Originally, the term was confined to light scattering. As more "ray"-like phenomena were discovered, the idea of scattering was extended to them, so that William Herschel could refer to the scattering of "heat rays" in 1800. John Tyndall, a pioneer in light scattering research, noted the connection between light scattering and acoustic scattering in the 1870s. Near the end of the 19th century, the scattering of cathode rays and X-rays was observed and discussed. With the discovery of subatomic particles and the development of quantum theory in the 20th century, the sense of the term became broader as it was recognized that the same mathematical frameworks used in light scattering could be applied to many other phenomena.
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial variables and is typically denoted as either or . The density is determined, through definition, by the normalised -electron wavefunction which itself depends upon variables. Conversely, the density determines the wave function modulo up to a phase factor, providing the formal foundation of density functional theory.

A synchrotron light source is a source of electromagnetic radiation (EM) usually produced by a storage ring, for scientific and technical purposes. First observed in synchrotrons, synchrotron light is now produced by storage rings and other specialized particle accelerators, typically accelerating electrons. Once the high-energy electron beam has been generated, it is directed into auxiliary components such as bending magnets and insertion devices in storage rings and free electron lasers. These supply the strong magnetic fields perpendicular to the beam which are needed to convert high energy electrons into photons.

Electron-beam lithography is the practice of scanning a focused beam of electrons to draw custom shapes on a surface covered with an electron-sensitive film called a resist (exposing). The electron beam changes the solubility of the resist, enabling selective removal of either the exposed or non-exposed regions of the resist by immersing it in a solvent (developing). The purpose, as with photolithography, is to create very small structures in the resist that can subsequently be transferred to the substrate material, often by etching.
High resolution electron energy loss spectroscopy (HREELS) is a tool used in surface science. The inelastic scattering of electrons from surfaces is utilized to study electronic excitations or vibrational modes of the surface of a material or of molecules adsorbed to a surface. In contrast to other electron energy loss spectroscopies (EELS), HREELS deals with small energy losses in the range of 10−3 eV to 1 eV. It plays an important role in the investigation of surface structure, catalysis, dispersion of surface phonons and the monitoring of epitaxial growth.
In mathematics and physics, scattering theory is a framework for studying and understanding the scattering of waves and particles. Wave scattering corresponds to the collision and scattering of a wave with some material object, for instance sunlight scattered by rain drops to form a rainbow. Scattering also includes the interaction of billiard balls on a table, the Rutherford scattering of alpha particles by gold nuclei, the Bragg scattering of electrons and X-rays by a cluster of atoms, and the inelastic scattering of a fission fragment as it traverses a thin foil. More precisely, scattering consists of the study of how solutions of partial differential equations, propagating freely "in the distant past", come together and interact with one another or with a boundary condition, and then propagate away "to the distant future". The direct scattering problem is the problem of determining the distribution of scattered radiation/particle flux basing on the characteristics of the scatterer. The inverse scattering problem is the problem of determining the characteristics of an object from measurement data of radiation or particles scattered from the object.
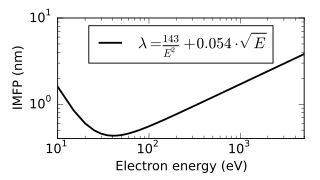
The inelastic mean free path (IMFP) is an index of how far an electron on average travels through a solid before losing energy.

Secondary electrons are electrons generated as ionization products. They are called 'secondary' because they are generated by other radiation. This radiation can be in the form of ions, electrons, or photons with sufficiently high energy, i.e. exceeding the ionization potential. Photoelectrons can be considered an example of secondary electrons where the primary radiation are photons; in some discussions photoelectrons with higher energy (>50 eV) are still considered "primary" while the electrons freed by the photoelectrons are "secondary".
Energy-filtered transmission electron microscopy (EFTEM) is a technique used in transmission electron microscopy, in which only electrons of particular kinetic energies are used to form the image or diffraction pattern. The technique can be used to aid chemical analysis of the sample in conjunction with complementary techniques such as electron crystallography.

Spartan is a molecular modelling and computational chemistry application from Wavefunction. It contains code for molecular mechanics, semi-empirical methods, ab initio models, density functional models, post-Hartree–Fock models, and thermochemical recipes including G3(MP2) and T1. Quantum chemistry calculations in Spartan are powered by Q-Chem.

Low-energy electron diffraction (LEED) is a technique for the determination of the surface structure of single-crystalline materials by bombardment with a collimated beam of low-energy electrons (30–200 eV) and observation of diffracted electrons as spots on a fluorescent screen.
X-ray absorption near edge structure (XANES), also known as near edge X-ray absorption fine structure (NEXAFS), is a type of absorption spectroscopy that indicates the features in the X-ray absorption spectra (XAS) of condensed matter due to the photoabsorption cross section for electronic transitions from an atomic core level to final states in the energy region of 50–100 eV above the selected atomic core level ionization energy, where the wavelength of the photoelectron is larger than the interatomic distance between the absorbing atom and its first neighbour atoms.
Surface stress was first defined by Josiah Willard Gibbs (1839-1903) as the amount of the reversible work per unit area needed to elastically stretch a pre-existing surface. A suggestion is surface stress define as association with the amount of the reversible work per unit area needed to elastically stretch a pre-existing surface instead of up definition. A similar term called "surface free energy", which represents the excess free energy per unit area needed to create a new surface, is easily confused with "surface stress". Although surface stress and surface free energy of liquid–gas or liquid–liquid interface are the same, they are very different in solid–gas or solid–solid interface, which will be discussed in details later. Since both terms represent a force per unit length, they have been referred to as "surface tension", which contributes further to the confusion in the literature.
Quantemol Ltd is based in University College London initiated by Professor Jonathan Tennyson FRS and Dr. Daniel Brown in 2004. The company initially developed a unique software tool, Quantemol-N, which provides full accessibility to the highly sophisticated UK molecular R-matrix codes, used to model electron polyatomic molecule interactions. Since then Quantemol has widened to further types of simulation, with plasmas and industrial plasma tools, in Quantemol-VT in 2013 and launched in 2016 a sustainable database Quantemol-DB, representing the chemical and radiative transport properties of a wide range of plasmas.
The charge-shift bond has been proposed as a new class of chemical bond that sits alongside the three familiar families of covalent, ionic bonds, and metallic bonds where electrons are shared or transferred respectively. The charge shift bond derives its stability from the resonance of ionic forms rather than the covalent sharing of electrons which are often depicted as having electron density between the bonded atoms. A feature of the charge shift bond is that the predicted electron density between the bonded atoms is low. It has long been known from experiment that the accumulation of electronic charge between the bonded atoms is not necessarily a feature of covalent bonds. An example where charge shift bonding has been used to explain the low electron density found experimentally is in the central bond between the inverted tetrahedral carbons in [1.1.1]propellanes. Theoretical calculations on a range of molecules have indicated that a charge shift bond is present, a striking example being fluorine, F2, which is normally described as having a typical covalent bond. The Charge Shift Bond(CSB) has also been shown to exist at the cation-anion interface of Protic Ionic Liquids(PILs). The authors have also shown how CSB character in PILs correlates with their physicochemical properties.










