Related Research Articles

Tannins are a class of astringent, polyphenolic biomolecules that bind to and precipitate proteins and various other organic compounds including amino acids and alkaloids.

In physics, chemistry, and materials science, percolation refers to the movement and filtering of fluids through porous materials. It is described by Darcy's law. Broader applications have since been developed that cover connectivity of many systems modeled as lattices or graphs, analogous to connectivity of lattice components in the filtration problem that modulates capacity for percolation.

Xanthan gum is a polysaccharide with many industrial uses, including as a common food additive. It is an effective thickening agent and stabilizer that prevents ingredients from separating. It can be produced from simple sugars by fermentation and derives its name from the species of bacteria used, Xanthomonas campestris.

A hydrogel is a biphasic material, a mixture of porous, permeable solids and at least 10% by weight or volume of interstitial fluid composed completely or mainly by water. In hydrogels the porous permeable solid is a water insoluble three dimensional network of natural or synthetic polymers and a fluid, having absorbed a large amount of water or biological fluids. These properties underpin several applications, especially in the biomedical area. Many hydrogels are synthetic, but some are derived from nature. The term 'hydrogel' was coined in 1894.
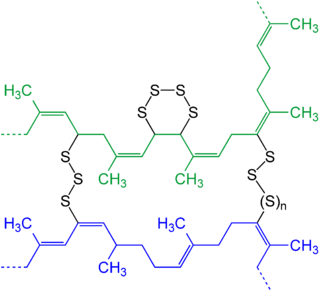
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural polymers.

Sodium polyacrylate (ACR, ASAP, or PAAS), also known as waterlock, is a sodium salt of polyacrylic acid with the chemical formula [−CH2−CH(CO2Na)−]n and has broad applications in consumer products. This super-absorbent polymer (SAP) has the ability to absorb 100 to 1000 times its mass in water. Sodium polyacrylate is an anionic polyelectrolyte with negatively charged carboxylic groups in the main chain. It is a polymer made up of chains of acrylate compounds. It contains sodium, which gives it the ability to absorb large amounts of water. When dissolved in water, it forms a thick and transparent solution due to the ionic interactions of the molecules. Sodium polyacrylate has many favorable mechanical properties. Some of these advantages include good mechanical stability, high heat resistance, and strong hydration. It has been used as an additive for food products including bread, juice, and ice cream.

Nanofibers are fibers with diameters in the nanometer range. Nanofibers can be generated from different polymers and hence have different physical properties and application potentials. Examples of natural polymers include collagen, cellulose, silk fibroin, keratin, gelatin and polysaccharides such as chitosan and alginate. Examples of synthetic polymers include poly(lactic acid) (PLA), polycaprolactone (PCL), polyurethane (PU), poly(lactic-co-glycolic acid) (PLGA), poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), and poly(ethylene-co-vinylacetate) (PEVA). Polymer chains are connected via covalent bonds. The diameters of nanofibers depend on the type of polymer used and the method of production. All polymer nanofibers are unique for their large surface area-to-volume ratio, high porosity, appreciable mechanical strength, and flexibility in functionalization compared to their microfiber counterparts.

Wet chemistry is a form of analytical chemistry that uses classical methods such as observation to analyze materials. It is called wet chemistry since most analyzing is done in the liquid phase. Wet chemistry is also called bench chemistry since many tests are performed at lab benches.
A molecularly imprinted polymer (MIP) is a polymer that has been processed using the molecular imprinting technique which leaves cavities in the polymer matrix with an affinity for a chosen "template" molecule. The process usually involves initiating the polymerization of monomers in the presence of a template molecule that is extracted afterwards, leaving behind complementary cavities. These polymers have affinity for the original molecule and have been used in applications such as chemical separations, catalysis, or molecular sensors. Published works on the topic date to the 1930s.

A superabsorbent polymer (SAP) (also called slush powder) is a water-absorbing hydrophilic homopolymers or copolymers that can absorb and retain extremely large amounts of a liquid relative to its own mass.
pH sensitive or pH responsive polymers are materials which will respond to the changes in the pH of the surrounding medium by varying their dimensions. Materials may swell, collapse, or change depending on the pH of their environment. This behavior is exhibited due to the presence of certain functional groups in the polymer chain. pH-sensitive materials can be either acidic or basic, responding to either basic or acidic pH values. These polymers can be designed with many different architectures for different applications. Key uses of pH sensitive polymers are controlled drug delivery systems, biomimetics, micromechanical systems, separation processes, and surface functionalization.
Radiation damage is the effect of ionizing radiation on physical objects including non-living structural materials. It can be either detrimental or beneficial for materials.
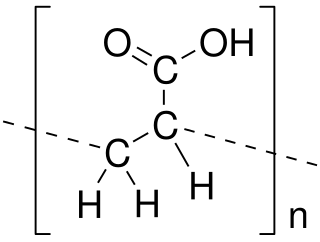
Poly(acrylic acid) (PAA; trade name Carbomer) is a polymer with the formula (CH2-CHCO2H)n. It is a derivative of acrylic acid (CH2=CHCO2H). In addition to the homopolymers, a variety of copolymers and crosslinked polymers, and partially deprotonated derivatives thereof are known and of commercial value. In a water solution at neutral pH, PAA is an anionic polymer, i.e., many of the side chains of PAA lose their protons and acquire a negative charge. Partially or wholly deprotonated PAAs are polyelectrolytes, with the ability to absorb and retain water and swell to many times their original volume. These properties – acid-base and water-attracting – are the bases of many applications.

Polyethylenimine (PEI) or polyaziridine is a polymer with repeating units composed of the amine group and two carbon aliphatic CH2CH2 spacers. Linear polyethyleneimines contain all secondary amines, in contrast to branched PEIs which contain primary, secondary and tertiary amino groups. Totally branched, dendrimeric forms were also reported. PEI is produced on an industrial scale and finds many applications usually derived from its polycationic character.
Membrane technology encompasses the scientific processes used in the construction and application of membranes. Membranes are used to facilitate the transport or rejection of substances between mediums, and the mechanical separation of gas and liquid streams. In the simplest case, filtration is achieved when the pores of the membrane are smaller than the diameter of the undesired substance, such as a harmful microorganism. Membrane technology is commonly used in industries such as water treatment, chemical and metal processing, pharmaceuticals, biotechnology, the food industry, as well as the removal of environmental pollutants.

Bacterial cellulose is an organic compound with the formula (C
6H
10O
5)
n produced by certain types of bacteria. While cellulose is a basic structural material of most plants, it is also produced by bacteria, principally of the genera Komagataeibacter, Acetobacter, Sarcina ventriculi and Agrobacterium. Bacterial, or microbial, cellulose has different properties from plant cellulose and is characterized by high purity, strength, moldability and increased water holding ability. In natural habitats, the majority of bacteria synthesize extracellular polysaccharides, such as cellulose, which form protective envelopes around the cells. While bacterial cellulose is produced in nature, many methods are currently being investigated to enhance cellulose growth from cultures in laboratories as a large-scale process. By controlling synthesis methods, the resulting microbial cellulose can be tailored to have specific desirable properties. For example, attention has been given to the bacteria Komagataeibacter xylinus due to its cellulose's unique mechanical properties and applications to biotechnology, microbiology, and materials science.
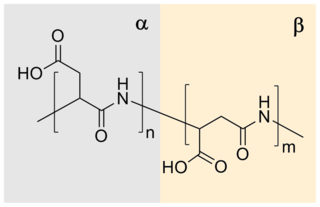
Polyaspartic acid (PASA) is a biodegradable, water-soluble condensation polymer based on the amino acid aspartic acid. It is a biodegradable replacement for water softeners and related applications. PASA can be chemically crosslinked with a wide variety of methods to yield PASA hydrogels. The resulting hydrogels are pH-sensitive such that under acidic conditions, they shrink, while the swelling capacity increases under alkaline conditions.
Common hydrogel agriculture's ingredient is potassium polyacrylate or sodium polyacrylate. As a superabsorbent material, it can absorb plenty of water and turn water to gel to store water.

Microneedles or Microneedle patches or Microarray patches are micron-scaled medical devices used to administer vaccines, drugs, and other therapeutic agents. While microneedles were initially explored for transdermal drug delivery applications, their use has been extended for the intraocular, vaginal, transungual, cardiac, vascular, gastrointestinal, and intracochlear delivery of drugs. Microneedles are constructed through various methods, usually involving photolithographic processes or micromolding. These methods involve etching microscopic structure into resin or silicon in order to cast microneedles. Microneedles are made from a variety of material ranging from silicon, titanium, stainless steel, and polymers. Some microneedles are made of a drug to be delivered to the body but are shaped into a needle so they will penetrate the skin. The microneedles range in size, shape, and function but are all used as an alternative to other delivery methods like the conventional hypodermic needle or other injection apparatus.

Self-healing concrete is characterized as the capability of concrete to fix its cracks on its own autogenously or autonomously. It not only seals the cracks but also partially or entirely recovers the mechanical properties of the structural elements. This kind of concrete is also known as self-repairing concrete. Because concrete has a poor tensile strength compared to other building materials, it often develops cracks in the surface. These cracks reduce the durability of the concrete because they facilitate the flow of liquids and gases that may contain harmful compounds. If microcracks expand and reach the reinforcement, not only will the concrete itself be susceptible to attack, but so will the reinforcement steel bars. Therefore, it is essential to limit the crack's width and repair it as quickly as feasible. Self-healing concrete would not only make the material more sustainable, but it would also contribute to an increase in the service life of concrete structures and make the material more durable and environmentally friendly.
References
- ↑ Anandan, Dhivyaa; Madhumathi, G.; Nambiraj, N. Arunai; Jaiswal, Amit K. (June 15, 2019). "Gum based 3D composite scaffolds for bone tissue engineering applications". Carbohydrate Polymers. 214: 62–70. doi:10.1016/j.carbpol.2019.03.020. ISSN 0144-8617. PMID 30926008. S2CID 88481556.
- ↑ Zhong, Peihua; Wang, Jun; Wang, Xiaoxian; Liu, Jiaping; Li, Zhen; Zhou, Yichuan (2020). "Comparison of Different Approaches for Testing Sorption by a Superabsorbent Polymer to Be Used in Cement-Based Materials". Materials. 13 (21): 5015. Bibcode:2020Mate...13.5015Z. doi: 10.3390/ma13215015 . ISSN 1996-1944. PMC 7664450 . PMID 33172166.
- ↑ Yang, Zijiang; Arakawa, Hisayuki (2023). "A beaker method for determination of microplastic concentration by micro-Raman spectroscopy". Methodsx. 11. doi:10.1016/j.mex.2023.102251. PMC 10336159 . PMID 37448948.