Related Research Articles
Nephrology is a specialty for both adult internal medicine and pediatric medicine that concerns the study of the kidneys, specifically normal kidney function and kidney disease, the preservation of kidney health, and the treatment of kidney disease, from diet and medication to renal replacement therapy. The word "renal" is an adjective meaning "relating to the kidneys", and its roots are French or late Latin. Whereas according to some opinions, "renal" and "nephro" should be replaced with "kidney" in scientific writings such as "kidney medicine" or "kidney replacement therapy", other experts have advocated preserving the use of renal and nephro as appropriate including in "nephrology" and "renal replacement therapy", respectively.

William Castle DeVries is an American cardiothoracic surgeon, mainly known for the first transplant of a TAH using the Jarvik-7 model.
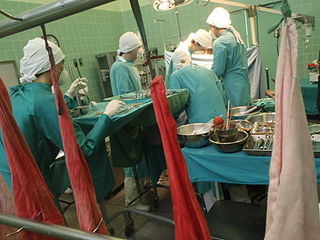
Organ transplantation is a medical procedure in which an organ is removed from one body and placed in the body of a recipient, to replace a damaged or missing organ. The donor and recipient may be at the same location, or organs may be transported from a donor site to another location. Organs and/or tissues that are transplanted within the same person's body are called autografts. Transplants that are recently performed between two subjects of the same species are called allografts. Allografts can either be from a living or cadaveric source.

An artificial heart is an artificial organ device that replaces the heart. Artificial hearts are typically used to bridge the time to complete heart transplantation surgery, but research is ongoing to develop a device that could permanently replace the heart in the case that a heart transplant is unavailable or not viable. As of December 2023, there are two commercially available full artificial heart devices; in both cases, they are for temporary use, of less than a year, for total heart failure patients awaiting a human heart to be transplanted into their bodies.

Liver transplantation or hepatic transplantation is the replacement of a diseased liver with the healthy liver from another person (allograft). Liver transplantation is a treatment option for end-stage liver disease and acute liver failure, although availability of donor organs is a major limitation. The most common technique is orthotopic transplantation, in which the native liver is removed and replaced by the donor organ in the same anatomic position as the original liver. The surgical procedure is complex, requiring careful harvest of the donor organ and meticulous implantation into the recipient. Liver transplantation is highly regulated, and only performed at designated transplant medical centers by highly trained transplant physicians and supporting medical team. Favorable outcomes require careful screening for eligible recipients, as well as a well-calibrated live or deceased donor match.
AbioCor was a total artificial heart (TAH) developed by the Massachusetts-based company AbioMed. It was fully implantable within a patient, due to a combination of advances in miniaturization, biosensors, plastics and energy transfer. The AbioCor ran on a rechargeable source of power. The internal battery was charged by a transcutaneous energy transmission (TET) system, meaning that no wires or tubes penetrated the skin, reducing the risk of infection. However, because of its size, this heart was only compatible with men who had a large frame. It had a product life expectancy of 18 months.

A ventricular assist device (VAD) is an electromechanical device that provides support for cardiac circulation, which is used either to partially or to completely replace the function of a failing heart. VADs can be used in patients with acute or chronic heart failure, which can occur due to a variety of reasons.
Abiomed, Inc. is a medical device technology company that operates as a stand-alone business within Johnson & Johnson's MedTech Segment. Abiomed develops and manufactures temporary external and implantable mechanical circulatory support devices. The company is headquartered in Danvers, Massachusetts with additional offices in Woburn, Baltimore, Berlin, Aachen, and Tokyo.

O. H. "Bud" Frazier is a heart surgeon and director of cardiovascular surgery research at the Texas Heart Institute (THI), best known for his work in mechanical circulatory support (MCS) of failing hearts using left ventricular assist devices (LVAD) and total artificial hearts (TAH).
An artificial lung (AL) is an artificial organ that provides oxygenation of blood and removal of carbon dioxide from the blood. The AL is intended to take over some of the functionality of biological lungs. It is different from a heart-lung machine in that it is external and designed to take over the functions of the lungs for long periods of time rather than on a temporary basis.
Transplantable organs and tissues may refer to both organs and tissues that are relatively often transplanted, as well as organs and tissues which are relatively seldom transplanted. In addition to this it may also refer to possible-transplants which are still in the experimental stage.
A wearable cardioverter defibrillator (WCD) is a non-invasive, external device for patients at risk of sudden cardiac arrest (SCA). It allows physicians time to assess their patient's arrhythmic risk and see if their ejection fraction improves before determining the next steps in patient care. It is a leased device. A summary of the device, its technology and indications was published in 2017 and reviewed by the EHRA Scientific Documents Committee.
Thoratec Corporation is a United States-based company that develops, manufactures, and markets proprietary medical devices used for mechanical circulatory support for the treatment of heart-failure patients worldwide. It is a global leader in mechanical circulatory support devices, particularly in ventricular assist devices (VADs).
Rodger Ford is the managing partner at Anthem Equity Group. He has also managed two medical device companies.
Wayne Robert Griffin is the second person in Australia to be implanted with the SynCardia Total artificial heart.

A heart transplant, or a cardiac transplant, is a surgical transplant procedure performed on patients with end-stage heart failure or severe coronary artery disease when other medical or surgical treatments have failed. As of 2018, the most common procedure is to take a functioning heart, with or without both lungs, from a recently deceased organ donor and implant it into the patient. The patient's own heart is either removed and replaced with the donor heart or, much less commonly, the recipient's diseased heart is left in place to support the donor heart.
ISMETT, in Italian, Istituto Mediterraneo per i Trapianti e Terapie ad Alta Specializzazione translated as the Mediterranean Institute for Transplantation and Advanced Specialized Therapies, is a center for organ transplantation located in Palermo, Italy. ISMETT was founded in 1997 as a partnership between the Region of Sicily, the Civico and Cervello hospitals in Palermo, and the University of Pittsburgh Medical Center (UPMC).

Limbal stem cells, also known as corneal epithelial stem cells, are unipotent stem cells located in the basal epithelial layer of the corneal limbus. They form the border between the cornea and the sclera. Characteristics of limbal stem cells include a slow turnover rate, high proliferative potential, clonogenicity, expression of stem cell markers, as well as the ability to regenerate the entire corneal epithelium. Limbal stem cell proliferation has the role of maintaining the cornea; for example, by replacing cells that are lost via tears. Additionally, these cells also prevent the conjunctival epithelial cells from migrating onto the surface of the cornea.
Jack Greene Copeland is an American cardiothoracic surgeon, who has established procedures in heart transplantation including repeat heart transplantation, the implantation of total artificial hearts (TAH) to bridge the time to heart transplant, innovations in left ventricular assist devices (LVAD) and the technique of "piggybacking" a second heart in a person, while leaving them the original.
Kevin K. Cheng, developed the Phoenix total artificial heart, first used in a human in 1985 in an emergency in a person whose donor heart was being acutely rejected. Cheng was a dentist by profession and the Phoenix heart he designed was originally developed to be implanted into a young cow. The recipient Thomas Creighton, who was dying from a failing heart, survived the artificial heart operation and 11 hours later a human heart was transplanted. The cause of death less than two days afterwards was not due to the artificial heart.
References
- 1 2 McKellar, Shelley (2018). Artificial Hearts: The Allure and Ambivalence of a Controversial Medical Technology. Johns Hopkins University. p. 315. ISBN 978-1421423555.
- 1 2 3 "About SynCardia". SynCardia. Archived from the original on 10 April 2020. Retrieved 6 November 2019.
- ↑ Elliott, Tonya; Sweet, Leslie C.; Wolfe, Alan C. (2018). "21. Mechanical circulatory support devices in transport". In Holleran, Reneé Semonin; Wolfe, Allen C.; Frakes, Michael A. (eds.). Patient Transport: Principles and Practice. Missouri: Elsevier. p. 337. ISBN 978-0-323-40110-4.
- ↑ Chakraborthy, Srijanee (20 September 2016). "Versa Capital Management acquires medical technology firm SynCardia". Verdict Medical Devices. Retrieved 26 January 2019.
- ↑ "New Documentary Traces History of SynCardia Total Artificial Heart". DAIC. 24 March 2016. Retrieved 26 January 2019.
- ↑ "SynCardia Welcomes New CEO". Medical Product Outsourcing. Retrieved 6 November 2019.