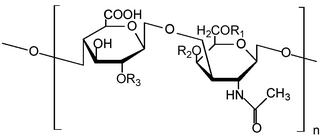
Glycosaminoglycans (GAGs) or mucopolysaccharides are long, linear polysaccharides consisting of repeating disaccharide units. The repeating two-sugar unit consists of a uronic sugar and an amino sugar, except in the case of the sulfated glycosaminoglycan keratan, where, in place of the uronic sugar there is a galactose unit. GAGs are found in vertebrates, invertebrates and bacteria. Because GAGs are highly polar molecules and attract water; the body uses them as lubricants or shock absorbers.

P-selectin is a type-1 transmembrane protein that in humans is encoded by the SELP gene.

Versican is a large extracellular matrix proteoglycan that is present in a variety of human tissues. It is encoded by the VCAN gene.
Perlecan (PLC) also known as basement membrane-specific heparan sulfate proteoglycan core protein (HSPG) or heparan sulfate proteoglycan 2 (HSPG2), is a protein that in humans is encoded by the HSPG2 gene. The HSPG2 gene codes for a 4,391 amino acid protein with a molecular weight of 468,829. It is one of the largest known proteins. The name perlecan comes from its appearance as a "string of pearls" in rotary shadowed images.

Pleiotrophin (PTN) also known as heparin-binding brain mitogen (HBBM) or heparin-binding growth factor 8 (HBGF-8) or neurite growth-promoting factor 1 (NEGF1) or heparin affinity regulatory peptide (HARP) or heparin binding growth associated molecule (HB-GAM) is a protein that in humans is encoded by the PTN gene. Pleiotrophin is an 18-kDa growth factor that has a high affinity for heparin. It is structurally related to midkine and retinoic acid induced heparin-binding protein.

Heparan sulfate (HS) is a linear polysaccharide found in all animal tissues. It occurs as a proteoglycan in which two or three HS chains are attached in close proximity to cell surface or extracellular matrix proteins. In this form, HS binds to a variety of protein ligands, including Wnt, and regulates a wide range of biological activities, including developmental processes, angiogenesis, blood coagulation, abolishing detachment activity by GrB, and tumour metastasis. HS has also been shown to serve as cellular receptor for a number of viruses, including the respiratory syncytial virus. One study suggests that cellular heparan sulfate has a role in SARS-CoV-2 Infection, particularly when the virus attaches with ACE2.

Lacritin is a 12.3 kDa glycoprotein encoded in humans by the LACRT gene. Lacritin's discovery emerged from a screen for factors that stimulate tear protein secretion. Lacritin is a secreted protein found in tears and saliva. Lacritin also promotes tear secretion, the proliferation and survival of epithelial cells, and corneal wound healing Lacritin is thus a multifunctional prosecretory mitogen with cell survival activity. Natural or bacterial cleavage of lacritin releases a C-terminal fragment that is bactericidal.

Betaglycan also known as Transforming growth factor beta receptor III (TGFBR3), is a cell-surface chondroitin sulfate / heparan sulfate proteoglycan >300 kDa in molecular weight. Betaglycan binds to various members of the TGF-beta superfamily of ligands via its core protein, and bFGF via its heparan sulfate chains. TGFBR3 is the most widely expressed type of TGF-beta receptor. Its affinity towards all individual isoforms of TGF-beta is similarly high and therefore it plays an important role as a coreceptor mediating the binding of TGF-beta to its other receptors - specifically TGFBR2. The intrinsic kinase activity of this receptor has not yet been described. In regard of TGF-beta signalling it is generally considered a non-signaling receptor or a coreceptor. By binding to various member of the TGF-beta superfamily at the cell surface it acts as a reservoir of TGF-beta.

Syndecan 1 is a protein which in humans is encoded by the SDC1 gene. The protein is a transmembrane heparan sulfate proteoglycan and is a member of the syndecan proteoglycan family. The syndecan-1 protein functions as an integral membrane protein and participates in cell proliferation, cell migration and cell-matrix interactions via its receptor for extracellular matrix proteins. Syndecan-1 is a sponge for growth factors and chemokines, with binding largely via heparan sulfate chains. The syndecans mediate cell binding, cell signaling, and cytoskeletal organization and syndecan receptors are required for internalization of the HIV-1 tat protein.

Glypicans constitute one of the two major families of heparan sulfate proteoglycans, with the other major family being syndecans. Six glypicans have been identified in mammals, and are referred to as GPC1 through GPC6. In Drosophila two glypicans have been identified, and these are referred to as dally and dally-like. One glypican has been identified in C. elegans. Glypicans seem to play a vital role in developmental morphogenesis, and have been suggested as regulators for the Wnt and Hedgehog cell signaling pathways. They have additionally been suggested as regulators for fibroblast growth factor and bone morphogenic protein signaling.
Heparin-binding EGF-like growth factor (HB-EGF) is a member of the EGF family of proteins that in humans is encoded by the HBEGF gene.

Syndecan-2 is a protein that in humans is encoded by the SDC2 gene.

Syndecan-4 is a protein that in humans is encoded by the SDC4 gene. Syndecan-4 is one of the four vertebrate syndecans and has a molecular weight of ~20 kDa. Syndecans are the best-characterized plasma membrane proteoglycans. Their intracellular domain of membrane-spanning core protein interacts with actin cytoskeleton and signaling molecules in the cell cortex. Syndecans are normally found on the cell surface of fibroblasts and epithelial cells. Syndecans interact with fibronectin on the cell surface, cytoskeletal and signaling proteins inside the cell to modulate the function of integrin in cell-matrix adhesion. Also, syndecans bind to FGFs and bring them to the FGF receptor on the same cell. As a co-receptor or regulator, mutated certain proteoglycans could cause severe developmental defects, like disordered distribution or inactivation of signaling molecules.

Heparanase, also known as HPSE, is an enzyme that acts both at the cell-surface and within the extracellular matrix to degrade polymeric heparan sulfate molecules into shorter chain length oligosaccharides.

Syndecan-3 is a protein that in humans is encoded by the SDC3 gene.

Glypican-1 (GPC1) is a protein that in humans is encoded by the GPC1 gene. GPC1 is encoded by human GPC1 gene located at 2q37.3. GPC1 contains 558 amino acids with three predicted heparan sulfate chains.

Natural cytotoxicity triggering receptor 3 is a protein that in humans is encoded by the NCR3 gene. NCR3 has also been designated as CD337 and as NKp30. NCR3 belongs to the family of NCR membrane receptors together with NCR1 (NKp46) and NCR2 (NKp44).

Sulfatase 1, also known as SULF1, is an enzyme which in humans is encoded by the SULF1 gene.

Tumor necrosis factor (ligand) superfamily, member 12-member 13, also known as TNFSF12-TNFSF13, is a human gene.

Carbohydrate sulfotransferases are sulfotransferase enzymes that transfer sulfate to carbohydrate groups in glycoproteins and glycolipids. Carbohydrates are used by cells for a wide range of functions from structural purposes to extracellular communication. Carbohydrates are suitable for such a wide variety of functions due to the diversity in structure generated from monosaccharide composition, glycosidic linkage positions, chain branching, and covalent modification. Possible covalent modifications include acetylation, methylation, phosphorylation, and sulfation. Sulfation, performed by carbohydrate sulfotransferases, generates carbohydrate sulfate esters. These sulfate esters are only located extracellularly, whether through excretion into the extracellular matrix (ECM) or by presentation on the cell surface. As extracellular compounds, sulfated carbohydrates are mediators of intercellular communication, cellular adhesion, and ECM maintenance.

















