Related Research Articles

Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, classified according to the monomers used and the structure of the biopolymer formed: polynucleotides, polypeptides, and polysaccharides. The Polynucleotides, RNA and DNA, are long polymers of nucleotides. Polypeptides include proteins and shorter polymers of amino acids; some major examples include collagen, actin, and fibrin. Polysaccharides are linear or branched chains of sugar carbohydrates; examples include starch, cellulose, and alginate. Other examples of biopolymers include natural rubbers, suberin and lignin, cutin and cutan, melanin, and polyhydroxyalkanoates (PHAs).

Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegradation occurs under a specific set of circumstances.

Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle, including during their initial processing, use, disposal into the environment and recycling. The rate of this degradation varies significantly; biodegradation can take decades, whereas some industrial processes can completely decompose a polymer in hours.

Polyglycolide or poly(glycolic acid) (PGA), also spelled as polyglycolic acid, is a biodegradable, thermoplastic polymer and the simplest linear, aliphatic polyester. It can be prepared starting from glycolic acid by means of polycondensation or ring-opening polymerization. PGA has been known since 1954 as a tough fiber-forming polymer. Owing to its hydrolytic instability, however, its use has initially been limited. Currently polyglycolide and its copolymers (poly(lactic-co-glycolic acid) with lactic acid, poly(glycolide-co-caprolactone) with ε-caprolactone and poly (glycolide-co-trimethylene carbonate) with trimethylene carbonate) are widely used as a material for the synthesis of absorbable sutures and are being evaluated in the biomedical field.

Polycaprolactone (PCL) is a biodegradable polyester with a low melting point of around 60 °C and a glass transition temperature of about −60 °C. The most common use of polycaprolactone is in the production of speciality polyurethanes. Polycaprolactones impart good resistance to water, oil, solvent and chlorine to the polyurethane produced.
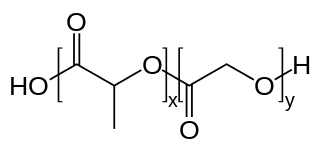
PLGA, PLG, or poly(lactic-co-glycolic acid) is a copolymer which is used in a host of Food and Drug Administration (FDA) approved therapeutic devices, owing to its biodegradability and biocompatibility. PLGA is synthesized by means of ring-opening co-polymerization of two different monomers, the cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid. Polymers can be synthesized as either random or block copolymers thereby imparting additional polymer properties. Common catalysts used in the preparation of this polymer include tin(II) 2-ethylhexanoate, tin(II) alkoxides, or aluminum isopropoxide. During polymerization, successive monomeric units are linked together in PLGA by ester linkages, thus yielding a linear, aliphatic polyester as a product.
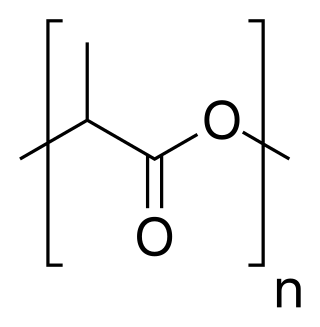
Polylactic acid, also known as poly(lactic acid) poly, lactic, acid or polylactide (PLA), is a thermoplastic polyester with backbone formula (C
3H
4O
2)
n or [–C(CH
3)HC(=O)O–]
n, formally obtained by condensation of lactic acid C(CH
3)(OH)HCOOH with loss of water. It can also be prepared by ring-opening polymerization of lactide [–C(CH
3)HC(=O)O–]
2, the cyclic dimer of the basic repeating unit.

Organ printing utilizes techniques similar to conventional 3D printing where a computer model is fed into a printer that lays down successive layers of plastics or wax until a 3D object is produced. In the case of organ printing, the material being used by the printer is a biocompatible plastic. The biocompatible plastic forms a scaffold that acts as the skeleton for the organ that is being printed. As the plastic is being laid down, it is also seeded with human cells from the patient's organ that is being printed for. After printing, the organ is transferred to an incubation chamber to give the cells time to grow. After a sufficient amount of time, the organ is implanted into the patient.
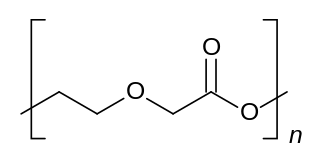
Polydioxanone or poly-p-dioxanone is a colorless, crystalline, biodegradable synthetic polymer.

Biodegradable plastics are plastics that can be decomposed by the action of living organisms, usually microbes, into water, carbon dioxide, and biomass. Biodegradable plastics are commonly produced with renewable raw materials, micro-organisms, petrochemicals, or combinations of all three.
A biomaterial is a substance that has been engineered to interact with biological systems for a medical purpose, either a therapeutic or a diagnostic one. As a science, biomaterials is about fifty years old. The study of biomaterials is called biomaterials science or biomaterials engineering. It has experienced steady and strong growth over its history, with many companies investing large amounts of money into the development of new products. Biomaterials science encompasses elements of medicine, biology, chemistry, tissue engineering and materials science.
Polyanhydrides are a class of biodegradable polymers characterized by anhydride bonds that connect repeat units of the polymer backbone chain. Their main application is in the medical device and pharmaceutical industry. In vivo, polyanhydrides degrade into non-toxic diacid monomers that can be metabolized and eliminated from the body. Owing to their safe degradation products, polyanhydrides are considered to be biocompatible.
Polymer engineering is generally an engineering field that designs, analyses, and modifies polymer materials. Polymer engineering covers aspects of the petrochemical industry, polymerization, structure and characterization of polymers, properties of polymers, compounding and processing of polymers and description of major polymers, structure property relations and applications.

Biodegradable polymers are a special class of polymer that breaks down after its intended purpose by bacterial decomposition process to result in natural byproducts such as gases (CO2, N2), water, biomass, and inorganic salts. These polymers are found both naturally and synthetically made, and largely consist of ester, amide, and ether functional groups. Their properties and breakdown mechanism are determined by their exact structure. These polymers are often synthesized by condensation reactions, ring opening polymerization, and metal catalysts. There are vast examples and applications of biodegradable polymers.

Poly(3-hydroxybutyrate-co-3-hydroxyvalerate), commonly known as PHBV, is a polyhydroxyalkanoate-type polymer. It is biodegradable, nontoxic, biocompatible plastic produced naturally by bacteria and a good alternative for many non-biodegradable synthetic polymers. It is a thermoplastic linear aliphatic polyester. It is obtained by the copolymerization of 3-hydroxybutanoic acid and 3-hydroxypentanoic acid. PHBV is used in speciality packaging, orthopedic devices and in controlled release of drugs. PHBV undergoes bacterial degradation in the environment.

Trimethylene carbonate, or 1,3-propylene carbonate, is a 6-membered cyclic carbonate ester. It is a colourless solid that upon heating or catalytic ring-opening converts to poly(trimethylene carbonate) (PTMC). Such polymers are called aliphatic polycarbonates and are of interest for potential biomedical applications. An isomeric derivative is propylene carbonate, a colourless liquid that does not spontaneously polymerize.
Bioresorbablemetals are metals or their alloys that degrade safely within the body. The primary metals in this category are magnesium-based and iron-based alloys, although recently zinc has also been investigated. Currently, the primary uses of bioresorbable metals are as stents for blood vessels and other internal ducts.
Polyorthoesters are polymers with the general structure –[–R–O–C(R1, OR2)–O–R3–]n– whereas the residue R2 can also be part of a heterocyclic ring with the residue R. Polyorthoesters are formed by transesterification of orthoesters with diols or by polyaddition between a diol and a diketene acetal, such as 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane.

Poly(trimethylene carbonate) (PTMC) is an aliphatic polycarbonate synthesized from the 6-membered cyclic carbonate, trimethylene carbonate (1,3-propylene carbonate or 1,3-Dioxan-2-one). Trimethylene carbonate (TMC) is a colorless crystalline solid with melting point ranging between 45°C and 48 °C and boiling point at 255ºC (at 760 mmHg). TMC is originally synthesized from 1,3-propanediol with phosgene or carbon monoxide, which are highly poisonous gases. Another route is from the transesterification of 1,3-propanediol and dialkylcarbonates. This route is considered "greener" compared to the other one, since precursors can be obtained from renewable resources and carbon dioxide.

Drug eluting implants encompass a wide range of bioactive implants that can be placed in or near a tissue to provide a controlled, sustained or on demand release of drug while overcoming barriers associated with traditional oral and intravenous drug administration, such as limited bioavailability, metabolism, and toxicity. These implants can be used to treat location-specific and surrounding illness and commonly use 3-D printing technologies to achieve individualized implants for patients.
References
- ↑ Gilding, D.K.; Reed, A.M. (1979). "Biodegradable polymers for use in surgery—polyglycolic/poly(actic acid) homo- and copolymers: 1". Polymer. 20 (12): 1459. doi:10.1016/0032-3861(79)90009-0.
- ↑ Pietrzak, WS; Verstynen, ML; Sarver, DR (1997). "Bioabsorbable fixation devices: Status for the craniomaxillofacial surgeon". The Journal of Craniofacial Surgery. 8 (2): 92–6. doi:10.1097/00001665-199703000-00005. PMID 10332273. S2CID 43882928.
- ↑ Pietrzak, WS; Sarver, DR; Verstynen, ML (1997). "Bioabsorbable polymer science for the practicing surgeon". The Journal of Craniofacial Surgery. 8 (2): 87–91. doi:10.1097/00001665-199703000-00004. PMID 10332272. S2CID 11105323.
- ↑ Middleton, John C. and Tipton, Arthur J. (March 1998) Synthetic Biodegradable Polymers as Medical Devices, Medical Plastics and Biomaterials Magazine, Retrieved (2009-11-09)
- ↑ Kohn J, and Langer R, "Bioresorbable and Bioerodible Materials," in Biomaterials Science: An Introduction to Materials in Medicine, Ratner BD (ed.), New York, Academic Press, 2004 ISBN 0125824637, pp. 115 ff
- ↑ Adolfsson, Karin H.; Sjöberg, Ida; Höglund, Odd V.; Wattle, Ove; Hakkarainen, Minna (2 August 2021). "In Vivo Versus In Vitro Degradation of a 3D Printed Resorbable Device for Ligation of Vascular Tissue in Horses". Macromolecular Bioscience. 21 (10): 2100164. doi: 10.1002/mabi.202100164 . PMID 34339098.
- ↑ Barret (2020-07-09). "Biodegradable Polymers for Drug Delivery". Polylactide and Polycaprolactone Manufacturer. Retrieved 2023-06-04.