
Technetium is a chemical element; it has symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. Technetium and promethium are the only radioactive elements whose neighbours in the sense of atomic number are both stable. All available technetium is produced as a synthetic element. Naturally occurring technetium is a spontaneous fission product in uranium ore and thorium ore, or the product of neutron capture in molybdenum ores. This silvery gray, crystalline transition metal lies between manganese and rhenium in group 7 of the periodic table, and its chemical properties are intermediate between those of both adjacent elements. The most common naturally occurring isotope is 99Tc, in traces only.
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (t1/2) for that collection, can be calculated from their measured decay constants. The range of the half-lives of radioactive atoms has no known limits and spans a time range of over 55 orders of magnitude.
A synthetic radioisotope is a radionuclide that is not found in nature: no natural process or mechanism exists which produces it, or it is so unstable that it decays away in a very short period of time. Examples include technetium-99 and promethium-146. Many of these are found in, and harvested from, spent nuclear fuel assemblies. Some must be manufactured in particle accelerators.
A radioactive tracer, radiotracer, or radioactive label is a synthetic derivative of a natural compound in which one or more atoms have been replaced by a radionuclide. By virtue of its radioactive decay, it can be used to explore the mechanism of chemical reactions by tracing the path that the radioisotope follows from reactants to products. Radiolabeling or radiotracing is thus the radioactive form of isotopic labeling. In biological contexts, experiments that use radioisotope tracers are sometimes called radioisotope feeding experiments.

Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy, and gamma rays. The two smaller nuclei are the fission products..

Radiopharmacology is radiochemistry applied to medicine and thus the pharmacology of radiopharmaceuticals. Radiopharmaceuticals are used in the field of nuclear medicine as radioactive tracers in medical imaging and in therapy for many diseases. Many radiopharmaceuticals use technetium-99m (Tc-99m) which has many useful properties as a gamma-emitting tracer nuclide. In the book Technetium a total of 31 different radiopharmaceuticals based on Tc-99m are listed for imaging and functional studies of the brain, myocardium, thyroid, lungs, liver, gallbladder, kidneys, skeleton, blood and tumors.
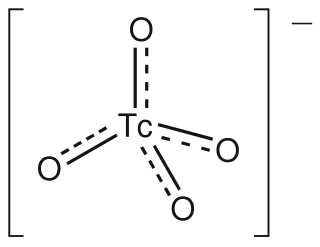
The pertechnetate ion is an oxyanion with the chemical formula TcO−
4. It is often used as a convenient water-soluble source of isotopes of the radioactive element technetium (Tc). In particular it is used to carry the 99mTc isotope which is commonly used in nuclear medicine in several nuclear scanning procedures.

There are 37 known isotopes of iodine (53I) from 108I to 144I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element.
Technetium (43Tc) is one of the two elements with Z < 83 that have no stable isotopes; the other such element is promethium. It is primarily artificial, with only trace quantities existing in nature produced by spontaneous fission or neutron capture by molybdenum. The first isotopes to be synthesized were 97Tc and 99Tc in 1936, the first artificial element to be produced. The most stable radioisotopes are 97Tc, 98Tc, and 99Tc.
Molybdenum (42Mo) has 39 known isotopes, ranging in atomic mass from 81 to 119, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. All unstable isotopes of molybdenum decay into isotopes of zirconium, niobium, technetium, and ruthenium.
Naturally occurring zirconium (40Zr) is composed of four stable isotopes (of which one may in the future be found radioactive), and one very long-lived radioisotope (96Zr), a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay, which is not yet observed, but the theoretically predicted value of t1/2 is 2.4×1020 years. The second most stable radioisotope is 93Zr, which has a half-life of 1.53 million years. Thirty other radioisotopes have been observed. All have half-lives less than a day except for 95Zr (64.02 days), 88Zr (83.4 days), and 89Zr (78.41 hours). The primary decay mode is electron capture for isotopes lighter than 92Zr, and the primary mode for heavier isotopes is beta decay.
Plutonium (94Pu) is an artificial element, except for trace quantities resulting from neutron capture by uranium, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. It was synthesized long before being found in nature, the first isotope synthesized being plutonium-238 in 1940. Twenty plutonium radioisotopes have been characterized. The most stable are plutonium-244 with a half-life of 80.8 million years; plutonium-242 with a half-life of 373,300 years; and plutonium-239 with a half-life of 24,110 years; and plutonium-240 with a half-life of 6,560 years. This element also has eight meta states; all have half-lives of less than one second.

This page discusses each of the main elements in the mixture of fission products produced by nuclear fission of the common nuclear fuels uranium and plutonium. The isotopes are listed by element, in order by atomic number.

Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99, symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used medical radioisotope in the world.
Technetium-99 (99Tc) is an isotope of technetium which decays with a half-life of 211,000 years to stable ruthenium-99, emitting beta particles, but no gamma rays. It is the most significant long-lived fission product of uranium fission, producing the largest fraction of the total long-lived radiation emissions of nuclear waste. Technetium-99 has a fission product yield of 6.0507% for thermal neutron fission of uranium-235.
Long-lived fission products (LLFPs) are radioactive materials with a long half-life produced by nuclear fission of uranium and plutonium. Because of their persistent radiotoxicity, it is necessary to isolate them from humans and the biosphere and to confine them in nuclear waste repositories for geological periods of time. The focus of this article is radioisotopes (radionuclides) generated by fission reactors.

In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the interstellar medium from which the solar system was formed, and were formed in, or after, the Big Bang, by nucleosynthesis in stars and supernovae followed by mass ejection, by cosmic ray spallation, and potentially from other processes. They are the stable nuclides plus the long-lived fraction of radionuclides surviving in the primordial solar nebula through planet accretion until the present; 286 such nuclides are known.

Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.
Phoenix, formerly known as Phoenix Nuclear Labs, is a company specializing in neutron generator technology located in Monona, Wisconsin. Founded in 2005, the company develops nuclear and particle accelerator technologies for application in medicine, defense and energy. Phoenix has held contracts with the U.S. Army, the U.S. Department of Energy, the U.S. Department of Defense and the U.S. Air Force. Phoenix developed a proprietary gas target neutron generator technology and has designed and built a number of particle accelerator-related technologies.









