Infertility is the inability of a person, animal or plant to reproduce by natural means. It is usually not the natural state of a healthy adult, except notably among certain eusocial species. It is the normal state of a human child or other young offspring, because they have not undergone puberty, which is the body's start of reproductive capacity.
Andrology is a name for the medical specialty that deals with male health, particularly relating to the problems of the male reproductive system and urological problems that are unique to men. It is the counterpart to gynaecology, which deals with medical issues which are specific to female health, especially reproductive and urologic health.

Testicular cancer is cancer that develops in the testicles, a part of the male reproductive system. Symptoms may include a lump in the testicle or swelling or pain in the scrotum. Treatment may result in infertility.

Cryptorchidism, also known as undescended testis, is the failure of one or both testes to descend into the scrotum. The word is from Greek κρυπτός 'hidden' and ὄρχις 'testicle'. It is the most common birth defect of the male genital tract. About 3% of full-term and 30% of premature infant boys are born with at least one undescended testis. However, about 80% of cryptorchid testes descend by the first year of life, making the true incidence of cryptorchidism around 1% overall. Cryptorchidism may develop after infancy, sometimes as late as young adulthood, but that is exceptional.

Spermatogenesis is the process by which haploid spermatozoa develop from germ cells in the seminiferous tubules of the testis. This process starts with the mitotic division of the stem cells located close to the basement membrane of the tubules. These cells are called spermatogonial stem cells. The mitotic division of these produces two types of cells. Type A cells replenish the stem cells, and type B cells differentiate into primary spermatocytes. The primary spermatocyte divides meiotically into two secondary spermatocytes; each secondary spermatocyte divides into two equal haploid spermatids by Meiosis II. The spermatids are transformed into spermatozoa (sperm) by the process of spermiogenesis. These develop into mature spermatozoa, also known as sperm cells. Thus, the primary spermatocyte gives rise to two cells, the secondary spermatocytes, and the two secondary spermatocytes by their subdivision produce four spermatozoa and four haploid cells.

Persistent Müllerian duct syndrome (PMDS) is the presence of Müllerian duct derivatives in what would be considered a genetically and otherwise physically normal male animal by typical human based standards. In humans, PMDS typically is due to an autosomal recessive congenital disorder and is considered by some to be a form of pseudohermaphroditism due to the presence of Müllerian derivatives.

Anogenital distance (AGD) is the distance from the midpoint of the anus to the genitalia, the underside of the vagina, the clitoris or the scrotum. It is considered medically significant for a number of reasons, in both humans and other animals, including sex determination and as a marker of endocrine disruptor exposure. It is regulated by dihydrotestosterone, which can be disrupted by phthalates common in plastics.

Azoospermia is the medical condition of a man whose semen contains no sperm. It is associated with male infertility, but many forms are amenable to medical treatment. In humans, azoospermia affects about 1% of the male population and may be seen in up to 20% of male infertility situations in Canada.
Terms oligospermia, oligozoospermia, and low sperm count refer to semen with a low concentration of sperm and is a common finding in male infertility. Often semen with a decreased sperm concentration may also show significant abnormalities in sperm morphology and motility. There has been interest in replacing the descriptive terms used in semen analysis with more quantitative information.
Male infertility refers to a sexually mature male's inability to impregnate a fertile female. In humans it accounts for 40–50% of infertility. It affects approximately 7% of all men. Male infertility is commonly due to deficiencies in the semen, and semen quality is used as a surrogate measure of male fecundity. More recently, advance sperm analyses that examine intracellular sperm components are being developed.
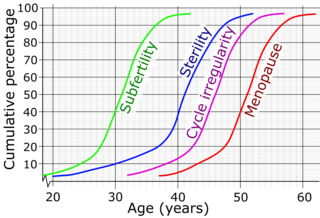
Female infertility refers to infertility in women. It affects an estimated 48 million women, with the highest prevalence of infertility affecting women in South Asia, Sub-Saharan Africa, North Africa/Middle East, and Central/Eastern Europe and Central Asia. Infertility is caused by many sources, including nutrition, diseases, and other malformations of the uterus. Infertility affects women from around the world, and the cultural and social stigma surrounding it varies.

XX male syndrome, also known as de la Chapelle syndrome, is a rare congenital intersex condition in which an individual with a 46,XX karyotype has phenotypically male characteristics that can vary among cases. Synonyms include 46,XX testicular difference of sex development, 46,XX sex reversal, nonsyndromic 46,XX testicular DSD, and XX sex reversal.

Gonadal dysgenesis is classified as any congenital developmental disorder of the reproductive system in humans. It is atypical development of gonads in an embryo,. One type of gonadal dysgenesis is the development of functionless, fibrous tissue, termed streak gonads, instead of reproductive tissue. Streak gonads are a form of aplasia, resulting in hormonal failure that manifests as sexual infantism and infertility, with no initiation of puberty and secondary sex characteristics.

Reproductive medicine is a branch of medicine concerning the male and female reproductive systems. It encompasses a variety of reproductive conditions, their prevention and assessment, as well as their subsequent treatment and prognosis.
Semen quality is a measure of male fertility, a measure of the ability of sperm in semen to accomplish fertilization. Semen quality involves both sperm quantity and quality. Semen quality is a major factor for fertility.
Hypergonadotropic hypogonadism (HH), also known as primary or peripheral/gonadal hypogonadism or primary gonadal failure, is a condition which is characterized by hypogonadism which is due to an impaired response of the gonads to the gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), and in turn a lack of sex steroid production. As compensation and the lack of negative feedback, gonadotropin levels are elevated. Individuals with HH have an intact and functioning hypothalamus and pituitary glands so they are still able to produce FSH and LH. HH may present as either congenital or acquired, but the majority of cases are of the former nature. HH can be treated with hormone replacement therapy.
XXXYsyndrome is a genetic condition characterized by a sex chromosome aneuploidy, where individuals have two extra X chromosomes. People in most cases have two sex chromosomes: an X and a Y or two X chromosomes. The presence of one Y chromosome with a functioning SRY gene causes the expression of genes that determine maleness. Because of this, XXXY syndrome only affects males. The additional two X chromosomes in males with XXXY syndrome causes them to have 48 chromosomes, instead of the typical 46. XXXY syndrome is therefore often referred to as 48,XXXY. There is a wide variety of symptoms associated with this syndrome, including cognitive and behavioral problems, taurodontism, and infertility. This syndrome is usually inherited via a new mutation in one of the parents' gametes, as those affected by it are usually infertile. It is estimated that XXXY affects one in every 50,000 male births.
Diethylstilbestrol (DES), a synthetic nonsteroidal estrogen which was previously used clinically to support pregnancy, has been linked to a variety of long-term adverse effects in women who were treated with it during pregnancy and in their offspring.

The male infertility crisis is an increase in male infertility since the mid-1970s. The issue attracted media attention after a 2017 meta-analysis found that sperm counts had declined by 52.4 percent between 1973 and 2011. The decline is particularly prevalent in Western countries such as New Zealand and Australia, Europe and North America. A 2022 meta-analysis reported that this decline extends to non-Western countries, namely those in Asia, Africa, Central America, and South America. This meta-analysis also suggests that the decline in sperm counts may be accelerating.
Sexual anomalies, also known as sexual abnormalities, are a set of clinical conditions due to chromosomal, gonadal and/or genitalia variation. Individuals with congenital (inborn) discrepancy between sex chromosome, gonadal, and their internal and external genitalia are categorised as individuals with a disorder of sex development (DSD). Afterwards, if the family or individual wishes, they can partake in different management and treatment options for their conditions.














