
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors silicon and tin. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature.

The carbon group is a periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.

Clemens Alexander Winkler was a German chemist who discovered the element germanium in 1886, solidifying Dmitri Mendeleev's theory of periodicity.

Argyrodite is an uncommon silver germanium sulfide mineral with formula Ag8GeS6. The color is iron-black with a purplish tinge, and the luster metallic.
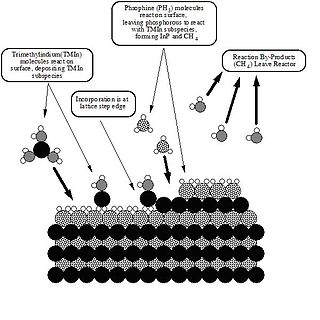
Metalorganic vapour-phase epitaxy (MOVPE), also known as organometallic vapour-phase epitaxy (OMVPE) or metalorganic chemical vapour deposition (MOCVD), is a chemical vapour deposition method used to produce single- or polycrystalline thin films. It is a process for growing crystalline layers to create complex semiconductor multilayer structures. In contrast to molecular-beam epitaxy (MBE), the growth of crystals is by chemical reaction and not physical deposition. This takes place not in vacuum, but from the gas phase at moderate pressures. As such, this technique is preferred for the formation of devices incorporating thermodynamically metastable alloys, and it has become a major process in the manufacture of optoelectronics, such as Light-emitting diodes. It was invented in 1968 at North American Aviation Science Center by Harold M. Manasevit.

Germane is the chemical compound with the formula GeH4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and water. Germane is a group 14 hydride.
Germanium dioxide, also called germanium(IV) oxide, germania, and salt of germanium, is an inorganic compound with the chemical formula GeO2. It is the main commercial source of germanium. It also forms as a passivation layer on pure germanium in contact with atmospheric oxygen.
Organogermanium chemistry is the science of chemical species containing one or more C–Ge bonds. Germanium shares group 14 in the periodic table with carbon, silicon, tin and lead. Historically, organogermanes are considered as nucleophiles and the reactivity of them is between that of organosilicon and organotin compounds. Some organogermanes have enhanced reactivity compared with their organosilicon and organoboron analogues in some cross-coupling reactions.
Winkler is a surname. Notable people with the surname include:

Germanium iodide is a chemical compound of germanium and iodine. Two such compounds exist: germanium(II) iodide, GeI2, and germanium(IV) iodide GeI4.

Silicon monoxide is the chemical compound with the formula SiO where silicon is present in the oxidation state +2. In the vapour phase, it is a diatomic molecule. It has been detected in stellar objects and has been described as the most common oxide of silicon in the universe.

Germanium disulfide or Germanium(IV) sulfide is the inorganic compound with the formula GeS2. It is a white high-melting crystalline solid. The compound is a 3-dimensional polymer, in contrast to silicon disulfide, which is a one-dimensional polymer. The Ge-S distance is 2.19 Å.

Germanium monoxide (chemical formula GeO) is a chemical compound of germanium and oxygen. It can be prepared as a yellow sublimate at 1000 °C by reacting GeO2 with Ge metal. The yellow sublimate turns brown on heating at 650 °C. GeO is not well characterised. It is amphoteric dissolving in acids to form germanium(II) salts and in alkali to form "trihydroxogermanates" or "germanites" containing the Ge(OH)3− ion.

Germanium dichloride is a chemical compound of germanium and chlorine with the formula GeCl2. It is a yellow solid. Germanium dichloride is an example of a compound featuring germanium in the +2 oxidation state.

Germanium tetrafluoride (GeF4) is a chemical compound of germanium and fluorine. It is a colorless gas.
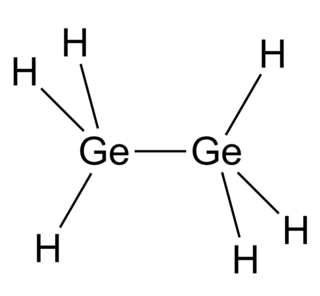
Digermane is an inorganic compound with the chemical formula Ge2H6. One of the few hydrides of germanium, it is a colourless liquid. Its molecular geometry is similar to ethane.

Germanium monosulfide or Germanium(II) sulfide is the chemical compound with the formula GeS. It is a chalcogenide glass and a semiconductor. Germanium sulfide is described as a red-brown powder or black crystals. Germanium(II) sulfide when dry is stable in air, hydrolyzes slowly in moist air but rapidly reacts in water forming Ge(OH)2 and then GeO. It is one of a few sulfides that can be sublimed under vacuum without decomposition.
Germanium(II) hydroxide, normally written as Ge(OH)2, is a poorly characterised compound, sometimes called hydrous germanium(II) oxide or germanous hydroxide. It was first reported by Winkler in 1886.

Germanium tetrabromide is an inorganic compound with the formula GeBr4. It can be formed by reacting solid germanium and gaseous bromine.
Germanium compounds are chemical compounds formed by the element germanium (Ge). Germanium is insoluble in dilute acids and alkalis but dissolves slowly in hot concentrated sulfuric and nitric acids and reacts violently with molten alkalis to produce germanates ([GeO
3]2−
). Germanium occurs mostly in the oxidation state +4 although many +2 compounds are known. Other oxidation states are rare: +3 is found in compounds such as Ge2Cl6, and +3 and +1 are found on the surface of oxides, or negative oxidation states in germanides, such as −4 in Mg
2Ge. Germanium cluster anions (Zintl ions) such as Ge42−, Ge94−, Ge92−, [(Ge9)2]6− have been prepared by the extraction from alloys containing alkali metals and germanium in liquid ammonia in the presence of ethylenediamine or a cryptand. The oxidation states of the element in these ions are not integers—similar to the ozonides O3−.


















