
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula [SiO(4-2x)−
4−x]
n, where 0 ≤ x < 2. The family includes orthosilicate SiO4−4, metasilicate SiO2−3, and pyrosilicate Si2O6−7. The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate [SiF6]2−.Most commonly, silicates are encountered as silicate minerals.
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases.

Thermogenin is a mitochondrial carrier protein found in brown adipose tissue (BAT). It is used to generate heat by non-shivering thermogenesis, and makes a quantitatively important contribution to countering heat loss in babies which would otherwise occur due to their high surface area-volume ratio.
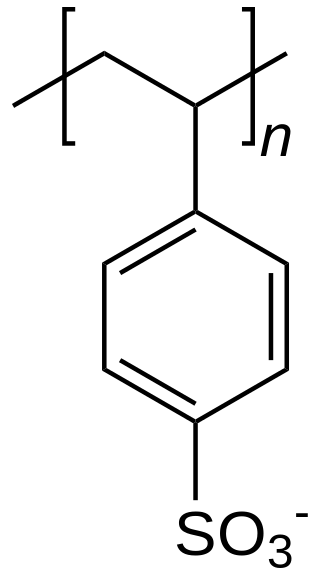
In chemistry, a counterion is the ion that accompanies an ionic species in order to maintain electric neutrality. In table salt the sodium ion is the counterion for the chloride ion and vice versa.
The anion gap is a value calculated from the results of multiple individual medical lab tests. It may be reported with the results of an electrolyte panel, which is often performed as part of a comprehensive metabolic panel.
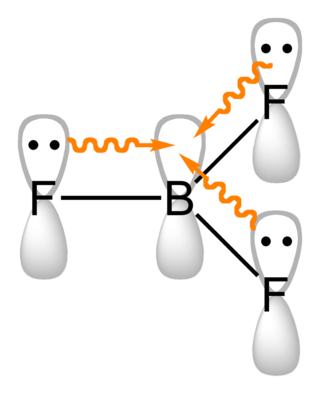
Boron compounds are compounds containing the element boron. In the most familiar compounds, boron has the formal oxidation state +3. These include oxides, sulfides, nitrides, and halides.

Excitatory amino-acid transporter 4 (EAAT4) is a protein that in humans is encoded by the SLC1A6 gene.

Potassium tetraphenylborate is the salt with the formula KB(C6H5)4). It is a colourless salt that is a rare example of a water-insoluble salt of potassium.

An uncoupling protein (UCP) is a mitochondrial inner membrane protein that is a regulated proton channel or transporter. An uncoupling protein is thus capable of dissipating the proton gradient generated by NADH-powered pumping of protons from the mitochondrial matrix to the mitochondrial intermembrane space. The energy lost in dissipating the proton gradient via UCPs is not used to do biochemical work. Instead, heat is generated. This is what links UCP to thermogenesis. However, not every type of UCPs are related to thermogenesis. Although UCP2 and UCP3 are closely related to UCP1, UCP2 and UCP3 do not affect thermoregulatory abilities of vertebrates. UCPs are positioned in the same membrane as the ATP synthase, which is also a proton channel. The two proteins thus work in parallel with one generating heat and the other generating ATP from ADP and inorganic phosphate, the last step in oxidative phosphorylation. Mitochondria respiration is coupled to ATP synthesis, but is regulated by UCPs. UCPs belong to the mitochondrial carrier (SLC25) family.

Mitochondrial uncoupling protein 2 is a protein that in humans is encoded by the UCP2 gene.

Mitochondrial uncoupling protein 3 is a protein that in humans is encoded by the UCP3 gene. The gene is located in chromosome (11q13.4) with an exon count of 7 and is expressed on the inner mitochondrial membrane. Uncoupling proteins transfer anions from the inner mitochondrial membrane to the outer mitochondrial membrane, thereby separating oxidative phosphorylation from synthesis of ATP, and dissipating energy stored in the mitochondrial membrane potential as heat. Uncoupling proteins also reduce generation of reactive oxygen species.

Brain mitochondrial carrier protein 1 is a protein that in humans is encoded by the SLC25A14 gene.

Mitochondrial uncoupling protein 4 is a protein that in humans is encoded by the SLC25A27 gene.
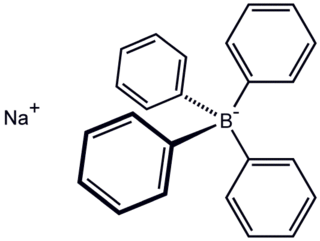
Sodium tetraphenylborate is the organic compound with the formula NaB(C6H5)4. It is a salt, wherein the anion consists of four phenyl rings bonded to boron. This white crystalline solid is used to prepare other tetraphenylborate salts, which are often highly soluble in organic solvents. The compound is used in inorganic and organometallic chemistry as a precipitating agent for potassium, ammonium, rubidium, and cesium ions, and some organic nitrogen compounds.
A protonophore, also known as a proton translocator, is an ionophore that moves protons across lipid bilayers or other type of membranes. This would otherwise not occur as protons cations (H+) have positive charge and hydrophilic properties, making them unable to cross without a channel or transporter in the form of a protonophore. Protonophores are generally aromatic compounds with a negative charge, that are both hydrophobic and capable of distributing the negative charge over a number of atoms by π-orbitals which delocalize a proton's charge when it attaches to the molecule. Both the neutral and the charged protonophore can diffuse across the lipid bilayer by passive diffusion and simultaneously facilitate proton transport. Protonophores uncouple oxidative phosphorylation via a decrease in the membrane potential of the inner membrane of mitochondria. They stimulate mitochondria respiration and heat production. Protonophores (uncouplers) are often used in biochemistry research to help explore the bioenergetics of chemiosmotic and other membrane transport processes. It has been reported that the protonophore has antibacterial activity by perturbing bacterial proton motive force.

An ion is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons.

Lithium tetrakis(pentafluorophenyl)borate is the lithium salt of the weakly coordinating anion (B(C6F5)4)−. Because of its weakly coordinating abilities, lithium tetrakis(pentafluorophenyl)borate makes it commercially valuable in the salt form in the catalyst composition for olefin polymerization reactions and in electrochemistry. It is a water-soluble compound. Its anion is closely related to the non-coordinating anion known as BARF. The tetrakis(pentafluorophenyl)borates have the advantage of operating on a one-to-one stoichiometric basis with Group IV transition metal polyolefin catalysts, unlike methylaluminoxane (MAO) which may be used in large excess.
An uncoupler or uncoupling agent is a molecule that disrupts oxidative phosphorylation in prokaryotes and mitochondria or photophosphorylation in chloroplasts and cyanobacteria by dissociating the reactions of ATP synthesis from the electron transport chain. The result is that the cell or mitochondrion expends energy to generate a proton-motive force, but the proton-motive force is dissipated before the ATP synthase can recapture this energy and use it to make ATP. Because the intracellular supply of protons is replenished, uncouplers actually stimulate cellular metabolism. Uncouplers are capable of transporting protons through mitochondrial and lipid membranes.
A chloride channel blocker is a type of drug which inhibits the transmission of ions (Cl−) through chloride channels.

Transition metal nitrile complexes are coordination compounds containing nitrile ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile.














