
Calico is a heavy plain-woven textile made from unbleached, and often not fully processed, cotton. It may also contain unseparated husk parts. The fabric is far coarser than muslin, but less coarse and thick than canvas or denim. However, it is still very cheap owing to its unfinished and undyed appearance.

Textile is an umbrella term that includes various fiber-based materials, including fibers, yarns, filaments, threads, different fabric types, etc. At first, the word "textiles" only referred to woven fabrics. However, weaving is not the only manufacturing method, and many other methods were later developed to form textile structures based on their intended use. Knitting and non-woven are other popular types of fabric manufacturing. In the contemporary world, textiles satisfy the material needs for versatile applications, from simple daily clothing to bulletproof jackets, spacesuits, and doctor's gowns.

Tie-dye is a term used to describe a number of resist dyeing techniques and the resulting dyed products of these processes. The process of tie-dye typically consists of folding, twisting, pleating, or crumpling fabric or a garment, before binding with string or rubber bands, followed by the application of dye or dyes. The manipulations of the fabric before the application of dye are called resists, as they partially or completely prevent ('resist') the applied dye from coloring the fabric. More sophisticated tie-dye may involve additional steps, including an initial application of dye before the resist, multiple sequential dyeing and resist steps, and the use of other types of resists and discharge.
Sulfur dyes are the most commonly used dyes manufactured for cotton in terms of volume. They are inexpensive, generally have good wash-fastness, and are easy to apply. Sulfur dyes are predominantly black, brown, and dark blue. Red sulfur dyes are unknown, although a pink or lighter scarlet color is available.

Dyeing is the application of dyes or pigments on textile materials such as fibers, yarns, and fabrics with the goal of achieving color with desired color fastness. Dyeing is normally done in a special solution containing dyes and particular chemical material. Dye molecules are fixed to the fiber by absorption, diffusion, or bonding with temperature and time being key controlling factors. The bond between the dye molecule and fiber may be strong or weak, depending on the dye used. Dyeing and printing are different applications; in printing, color is applied to a localized area with desired patterns. In dyeing, it is applied to the entire textile.

Cambric or batiste is a fine dense cloth. It is a lightweight plain-weave fabric, originally from the commune of Cambrai, woven greige, then bleached, piece-dyed, and often glazed or calendered. Initially it was made of linen; from the 18th and 19th centuries the term came to apply to cotton fabrics as well.

Textile manufacturing or textile engineering is a major industry. It is largely based on the conversion of fibre into yarn, then yarn into fabric. These are then dyed or printed, fabricated into cloth which is then converted into useful goods such as clothing, household items, upholstery and various industrial products.

Textile printing is the process of applying color to fabric in definite patterns or designs. In properly printed fabrics the colour is bonded with the fibre, so as to resist washing and friction. Textile printing is related to dyeing but in dyeing properly the whole fabric is uniformly covered with one colour, whereas in printing one or more colours are applied to it in certain parts only, and in sharply defined patterns.
The manufacture of textiles is one of the oldest of human technologies. To make textiles, the first requirement is a source of fiber from which a yarn can be made, primarily by spinning. The yarn is processed by knitting or weaving, which turns yarn into cloth. The machine used for weaving is the loom. For decoration, the process of colouring yarn or the finished material is dyeing. For more information of the various steps, see textile manufacturing.

A bleachfield or bleaching green was an open area used for spreading cloth on the ground to be purified and whitened by the action of the sunlight. Bleaching fields were usually found in and around mill towns in Great Britain and were an integral part of textile manufacture during the Industrial Revolution.

In textile manufacturing, finishing refers to the processes that convert the woven or knitted cloth into a usable material and more specifically to any process performed after dyeing the yarn or fabric to improve the look, performance, or "hand" (feel) of the finish textile or clothing. The precise meaning depends on context.
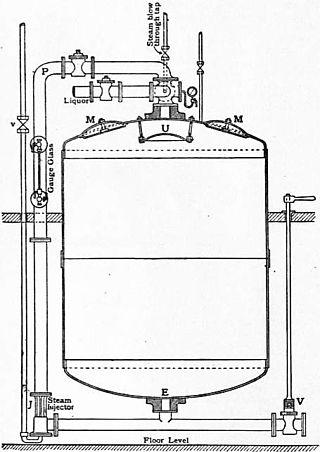
A kier or keeve is a large circular boiler or vat used in bleaching or scouring cotton fabric. They were also used for processing paper pulp.
Nantong blue calico printing and dyeing has been practiced in most parts of Nantong City, Jiangsu Province, China, since the beginning of Qing dynasty. In modern times, blue calico is used to make daily clothes, mosquito nets, pillowcases, baggage cloth, etc.
Wet Processing Engineering is one of the major streams in Textile Engineering or Textile manufacturing which refers to the engineering of textile chemical processes and associated applied science. The other three streams in textile engineering are yarn engineering, fabric engineering, and apparel engineering. The processes of this stream are involved or carried out in an aqueous stage. Hence, it is called a wet process which usually covers pre-treatment, dyeing, printing, and finishing.

Fabric treatments are processes that make fabric softer, or water resistant, or enhance dye penetration after they are woven. Fabric treatments get applied when the textile itself cannot add other properties. Treatments include, scrim, foam lamination, fabric protector or stain repellent, anti microbial and flame retardant.

Greige goods are loom state woven fabrics, or unprocessed knitted fabrics. Greige goods undergo many subsequent processes, for instance, dyeing, printing, bleaching, and finishing, prior to further converting to finished goods such as clothing, or other textile products. "Grey fabrics" is another term to refer to unfinished woven or knitted fabrics.

Grassing is one of the oldest methods of bleaching textile goods. The grassing method has been long been used in Europe to bleach linen and cotton based fabrics.

Scouring is a preparatory treatment of certain textile materials. Scouring removes soluble and insoluble impurities found in textiles as natural, added and adventitious impurities, for example, oils, waxes, fats, vegetable matter, as well as dirt. Removing these contaminants through scouring prepares the textiles for subsequent processes such as bleaching and dyeing. Though a general term, "scouring" is most often used for wool. In cotton, it is synonymously called "boiling out", and in silk, and "boiling off".

Discharge printing is a textile printing technique that involves the application of a discharging agent to strip dye from already-dyed cloth in order to produce a printed pattern, which can be either white or colored. It is a method to imprint a design onto dyed fabric. The print pattern is achieved by applying a substance capable of removing the color, such as chlorine or hydrosulfite, to create a white or light pattern on a darker-hued dyed background. A dischargeable dye is employed for dischargeable printing.
In textile processing, stripping is a color removal technique employed to partially or eliminate color from dyed textile materials. Textile dyeing industries often face challenges like uneven or flawed dyeing and the appearance of color patches on the fabric's surface during the dyeing process and subsequent textile material processing stages. Stripping is one of the reprocessing methods used to correct undesirable colors and flaws in dyed materials. The efficacy of this process relies on factors such as the dye type, fiber material, and the stripping agents utilized. Additionally, the procedure is recognized by alternative terms, namely back stripping or destructive stripping.



























