In computational chemistry and molecular physics, Gaussian orbitals are functions used as atomic orbitals in the LCAO method for the representation of electron orbitals in molecules and numerous properties that depend on these.

MOLPRO is a software package used for accurate ab initio quantum chemistry calculations. It is developed by Peter Knowles at Cardiff University and Hans-Joachim Werner at Universität Stuttgart in collaboration with other authors.
Oktay Sinanoğlu was a Turkish physical chemist and molecular biophysicist who made significant contributions to the theory of electron correlation in molecules, the statistical mechanics of clathrate hydrates, quantum chemistry, and the theory of solvation.
Semi-empirical quantum chemistry methods are based on the Hartree–Fock formalism, but make many approximations and obtain some parameters from empirical data. They are very important in computational chemistry for treating large molecules where the full Hartree–Fock method without the approximations is too expensive. The use of empirical parameters appears to allow some inclusion of electron correlation effects into the methods.

Hermann Hartmann was a German chemist and professor and researcher in physical and theoretical chemistry at the University of Frankfurt am Main. He contributed to all fields of physical chemistry and was instrumental in establishing theoretical chemistry by developing Ligand field theory (1947) and other quantum chemical models including the Hartmann Potential (1971). He also formulated a new perturbation theory (1970–1977) as part of his pioneering research towards a unified field theory of chemical bonding based on a non-linear Schrödinger equation (1980).

Karl James Jalkanen, FRSC,, is a research scientist in molecular biophysics. He is currently a research scientist at the Gilead Sciences new La Verne, California manufacturing facility in the Department of Technical Services.
Thomas R. Cundari is regents professor of chemistry at the University of North Texas and co-director of the Center for Advanced Scientific Computing and Modeling (CASCaM).

In graph theory, a caterpillar or caterpillar tree is a tree in which all the vertices are within distance 1 of a central path.
Sulfanyl (HS•), also known as the mercapto radical, hydrosulfide radical, or hydridosulfur, is a simple radical molecule consisting of one hydrogen and one sulfur atom. The radical appears in metabolism in organisms as H2S is detoxified. Sulfanyl is one of the top three sulfur-containing gasses in gas giants such as Jupiter and is very likely to be found in brown dwarfs and cool stars. It was originally discovered by Margaret N. Lewis and John U. White at the University of California in 1939. They observed molecular absorption bands around 325 nm belonging to the system designated by 2Σ+ ← 2Πi. They generated the radical by means of a radio frequency discharge in hydrogen sulfide. HS• is formed during the degradation of hydrogen sulfide in the atmosphere of the Earth. This may be a deliberate action to destroy odours or a natural phenomenon.

Donald Gene Truhlar is an American scientist working in theoretical and computational chemistry and chemical physics with special emphases on quantum mechanics and chemical dynamics.

Argonium (also called the argon hydride cation, the hydridoargon(1+) ion, or protonated argon; chemical formula ArH+) is a cation combining a proton and an argon atom. It can be made in an electric discharge, and was the first noble gas molecular ion to be found in interstellar space.
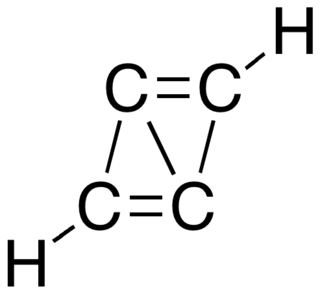
Propalene or bicyclo[1.1.0]buta-1,3-diene is a polycyclic hydrocarbon composed of two fused cyclopropene rings. Computational studies indicate that the molecule is planar, with the carbon framework forming a parallelogram that has distinctly alternating short and long carbon–carbon bonds.
Péter R. Surján is a Hungarian theoretical chemist who is known for his research on application of the theory of second quantization in quantum chemistry.
Quantum crystallography is a branch of crystallography that investigates crystalline materials within the framework of quantum mechanics, with analysis and representation, in position or in momentum space, of quantities like wave function, electron charge and spin density, density matrices and all properties related to them. Like the quantum chemistry, Quantum crystallography involves both experimental and computational work. The theoretical part of quantum crystallography is based on quantum mechanical calculations of atomic/molecular/crystal wave functions, density matrices or density models, used to simulate the electronic structure of a crystalline material. While in quantum chemistry, the experimental works mainly rely on spectroscopy, in quantum crystallography the scattering techniques play the central role, although spectroscopy as well as atomic microscopy are also sources of information.

Christopher J. Cramer is a research chemist and served as vice president for research at the University of Minnesota from 2018–2021. He presently serves as senior vice president and chief research officer for Underwriters Laboratories Inc.
The nitrate selenites are mixed anion compounds containing distinct nitrate (NO3−)and selenite (SO32−) groups. The compounds are colourless unless coloured by cations.
A nitrate nitrite, or nitrite nitrate, is a coordination complex or other chemical compound that contains both nitrite and nitrate anions (NO3− and NO2−). They are mixed-anion compounds, and they are mixed-valence compounds. Some have third anions. Many nitrite nitrate compounds are coordination complexes of cobalt. Such a substance was discovered by Wolcott Gibbs and Frederick Genth in 1857.
In computational chemistry, natural resonance theory (NRT) is an iterative, variational functional embedded into the natural bond orbital (NBO) program, commonly run in Gaussian, GAMESS, ORCA, Ampac and other software packages. NRT was developed in 1997 by Frank A. Weinhold and Eric D. Glendening, chemistry professors at University of Wisconsin-Madison and Indiana State University, respectively. Given a list of NBOs for an idealized natural Lewis structure, the NRT functional creates a list of Lewis resonance structures and calculates the resonance weights of each contributing resonance structure. Structural and chemical properties, such as bond order, valency, and bond polarity, may be calculated from resonance weights. Specifically, bond orders may be divided into their covalent and ionic contributions, while valency is the sum of bond orders of a given atom. This aims to provide quantitative results that agree with qualitative notions of chemical resonance. In contrast to the "wavefunction resonance theory" (i.e., the superposition of wavefunctions), NRT uses the density matrix resonance theory, performing a superposition of density matrices to realize resonance. NRT has applications in ab initio calculations, including calculating the bond orders of intra- and intermolecular interactions and the resonance weights of radical isomers.








