Related Research Articles

The chemical industry comprises the companies that develop and produce industrial, specialty and other chemicals. Central to the modern world economy, it converts raw materials into industrial and consumer products. The plastics industry contains some overlap, as some chemical companies produce plastics as well as chemicals.
In biochemistry, a disulfide refers to a functional group with the structure R−S−S−R′. The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. Persulfide usually refers to R−S−S−H compounds.

Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called epoxy. The IUPAC name for an epoxide group is an oxirane.

Phenol formaldehyde resins (PF) are synthetic polymers obtained by the reaction of phenol or substituted phenol with formaldehyde. Used as the basis for Bakelite, PFs were the first commercial synthetic resins (plastics). They have been widely used for the production of molded products including billiard balls, laboratory countertops, and as coatings and adhesives. They were at one time the primary material used for the production of circuit boards but have been largely replaced with epoxy resins and fiberglass cloth, as with fire-resistant FR-4 circuit board materials.
Sulfur dyes are the most commonly used dyes manufactured for cotton in terms of volume. They are inexpensive, generally have good wash-fastness, and are easy to apply. Sulfur dyes are predominantly black, brown, and dark blue. Red sulfur dyes are unknown, although a pink or lighter scarlet color is available.
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural polymers.
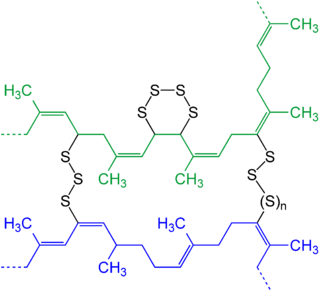
Polysulfides are a class of chemical compounds containing chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. Among the inorganic polysulfides, there are ones which contain anions, which have the general formula S2−
n. These anions are the conjugate bases of the hydrogen polysulfides H2Sn. Organic polysulfides generally have the formulae R1SnR2, where R = alkyl or aryl.
Thiokol was an American corporation concerned initially with rubber and related chemicals, and later with rocket and missile propulsion systems. Its name is a portmanteau of the Greek words for sulfur and glue, an allusion to the company's initial product, Thiokol polymer.

Sodium sulfide is a chemical compound with the formula Na2S, or more commonly its hydrate Na2S·9H2O. Both the anhydrous and the hydrated salts in pure crystalline form are colorless solids, although technical grades of sodium sulfide are generally yellow to brick red owing to the presence of polysulfides and commonly supplied as a crystalline mass, in flake form, or as a fused solid. They are water-soluble, giving strongly alkaline solutions. When exposed to moist air, Na2S and its hydrates emit hydrogen sulfide, an extremely toxic, flammable and corrosive gas which smells like rotten eggs.
Sulfur compounds are chemical compounds formed the element sulfur (S). Common oxidation states of sulfur range from −2 to +6. Sulfur forms stable compounds with all elements except the noble gases.
Silicone rubber is an elastomer composed of silicone—itself a polymer—containing silicon together with carbon, hydrogen, and oxygen. Silicone rubbers are widely used in industry, and there are multiple formulations. Silicone rubbers are often one- or two-part polymers, and may contain fillers to improve properties or reduce cost.
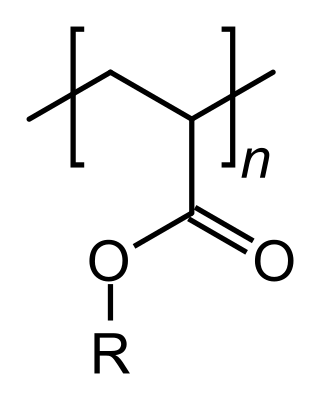
An acrylate polymer is any of a group of polymers prepared from acrylate monomers. These plastics are noted for their transparency, resistance to breakage, and elasticity.
Sealant is a substance used to block the passage of fluids through openings in materials, a type of mechanical seal. In building construction sealant is sometimes synonymous with caulk and also serve the purposes of blocking dust, sound and heat transmission. Sealants may be weak or strong, flexible or rigid, permanent or temporary. Sealants are not adhesives but some have adhesive qualities and are called adhesive-sealants or structural sealants.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.
Curing is a chemical process employed in polymer chemistry and process engineering that produces the toughening or hardening of a polymer material by cross-linking of polymer chains. Even if it is strongly associated with the production of thermosetting polymers, the term "curing" can be used for all the processes where a solid product is obtained from a liquid solution, such as with PVC plastisols.
RTV silicone is a type of silicone rubber that cures at room temperature. It is available as a one-component product, or mixed from two-components. Manufacturers provide it in a range of hardnesses from very soft to medium—usually from 15 to 40 Shore A. RTV silicones can be cured with a catalyst consisting of either platinum or a tin compound such as dibutyltin dilaurate. Applications include low-temperature over-molding, making molds for reproducing, and lens applications for some optically clear grades. It is also used widely in the automotive industry as an adhesive/sealant, for example to create gaskets in-place.

Sodium polysulfide is a general term for salts with the formula Na2Sx, where x = 2 to 5. The species Sx2−, called polysulfide anions, include disulfide (S22−), trisulfide (S32−), tetrasulfide (S42−), and pentasulfide (S52−). In principle, but not in practice, the chain lengths could be longer. The salts are dark red solids that dissolve in water to give highly alkaline and corrosive solutions. In air, these salts oxidize, and they evolve hydrogen sulfide by hydrolysis.
Sodium tetrasulfide is an inorganic compound with the formula Na2S4. It is a yellow-orange solid that dissolves via hydrolysis in water. It is a precursor to some specialty polymers and intermediates in prototypes of the sodium-sulfur battery.

Inverse vulcanization is a process that produces polysulfide polymers, which also contain some organic linkers. In contrast, sulfur vulcanization produces material that is predominantly organic but has a small percentage of polysulfide crosslinks.
2-Ethylhexyl glycidyl ether is a liquid organic molecule with formula C11H22O2 an industrial chemical used to reduce the viscosity of epoxy resins. These are then used in adhesives, sealants, and paints or coatings. It has the CAS Registry Number of 2461-15-6. It has the IUPAC name of 2-(2-ethylhexoxymethyl)oxirane. It also finds use in other polymer based applications.
References
- ↑ Mark S. M. Alger (1997). Polymer Science Dictionary. Springer. p. 569. ISBN 978-0-412-60870-4.
- 1 2 3 Vietti, David; Scherrer, Micheal (2000). "Polymers Containing Sulfur, Polysulfides". Kirk-Othmer Encyclopedia of Chemical Technology. New York: John Wiley. doi:10.1002/0471238961.1615122522090520.a01. ISBN 9780471238966.