
Thomas Loerting (born October 29, 1973) is an Austrian chemist and associate professor at the University of Innsbruck. His research focuses on amorphous systems, the physics and chemistry of ice and chemistry at low temperatures. [1]

Thomas Loerting (born October 29, 1973) is an Austrian chemist and associate professor at the University of Innsbruck. His research focuses on amorphous systems, the physics and chemistry of ice and chemistry at low temperatures. [1]
Thomas Loerting was born in Innsbruck where he also graduated from high-school ("Reithmanngymnasium") in 1992. He studied chemistry at the University of Innsbruck and received his Master's degree in 1997. In 1998, over the course of his Ph.D. in Innsbruck, Loerting was a guest lecturer at the Gadjah Mada University in Yogyakarta and the Chulalongkorn University in Bangkok, Thailand. He successfully defended his Ph.D. thesis titled "Kinetics of water mediated proton transfer in the atmosphere" in 2000. In 2001, he joined the team of Nobel laureate Mario Molina at the Massachusetts Institute of Technology. Loerting returned to Innsbruck in 2004 as assistant professor working together with Erwin Mayer. His habilitation "Disordered water at low temperatures" was submitted 2007. In 2008, he was elected as member of the Austrian Academy of Sciences ("Junge Kurie"). Since 2010, he is associate professor at the Institute of Physical Chemistry. [2] As of 2012, he is also speaker of the research platform "Advanced Materials", which counts over 10 research groups. [3] In addition to his academic work, he serves as advisor to the Austrian Luge Federation.
Thomas Loerting research includes more than 180 peer-reviewed international publications with more than 8000 citations, resulting in a h-index of 48 (as of October 2022). His first contributions were in the field of Theoretical and Computational chemistry, contributing to the understanding of hydration of sulfur dioxide and sulfur trioxide as well as the decomposition of chlorine nitrate, relevant to the chemistry of the atmosphere. He soon moved on to experiments where he advanced the field of amorphous ices, ice polymorphs and carbonic acid over the course of his career.
Thomas Loerting provided significant contributions for the understanding of polyamorphism in water. This includes the recognition of VHDA as third distinct amorphous state of water [4] as well as extensive studies on structure and dynamics of low- and high-density amorphous ice (LDA and HDA) using dilatometry, spectroscopy, calorimetry and diffraction. One particularly notable finding was that LDA and HDA both exhibit glass-to-liquid transitions at ambient pressure, [5] which provides support for the LLCP scenario in water. [6]
In addition to his achievements in the field of amorphous ices, Thomas Loerting is recognized for his works on crystalline ices, including the recent discovery of ice XIX. Together with his team he provided the first experimental proof that for each hydrogen-disordered ice phase (in this case: ice VI) several hydrogen-ordered counterparts (ices XV and XIX) may exist. [7] [8] [9] [10]
Another focus in the body of work of Thomas Loerting is acid-base chemistry under cryo-conditions. By employing the cryo-preparation and rapid quenching technique developed by Hage, Hallbrucker and Mayer, [11] coupled with FTIR-spectroscopy of the solid and the matrix isolated species, Loerting and his co-workers have pioneered formation and isomerisation of carbonic acid and its derivatives. [12] [13] [14] [15] [16] [17] [18] [19]
Thomas Loerting has received numerous awards for his scientific input. Among the most notable are:

In chemistry, a hydrogen bond is primarily an electrostatic force of attraction between a hydrogen (H) atom which is covalently bonded to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted Dn−H···Ac, where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the period 2 elements nitrogen (N), oxygen (O), and fluorine (F).

Carbonic acid is a chemical compound with the chemical formula H2CO3. The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion of carbon dioxide and carbonic acid is related to the breathing cycle of animals and the acidification of natural waters.

Clathrate hydrates, or gas hydrates, clathrates, or hydrates, are crystalline water-based solids physically resembling ice, in which small non-polar molecules or polar molecules with large hydrophobic moieties are trapped inside "cages" of hydrogen bonded, frozen water molecules. In other words, clathrate hydrates are clathrate compounds in which the host molecule is water and the guest molecule is typically a gas or liquid. Without the support of the trapped molecules, the lattice structure of hydrate clathrates would collapse into conventional ice crystal structure or liquid water. Most low molecular weight gases, including O2, H2, N2, CO2, CH4, H2S, Ar, Kr, and Xe, as well as some higher hydrocarbons and freons, will form hydrates at suitable temperatures and pressures. Clathrate hydrates are not officially chemical compounds, as the enclathrated guest molecules are never bonded to the lattice. The formation and decomposition of clathrate hydrates are first order phase transitions, not chemical reactions. Their detailed formation and decomposition mechanisms on a molecular level are still not well understood. Clathrate hydrates were first documented in 1810 by Sir Humphry Davy who found that water was a primary component of what was earlier thought to be solidified chlorine.

Ice XII is a metastable, dense, crystalline phase of solid water, a type of ice. Ice XII was first reported in 1996 by C. Lobban, J.L. Finney and W.F. Kuhs and, after initial caution, was properly identified in 1998.
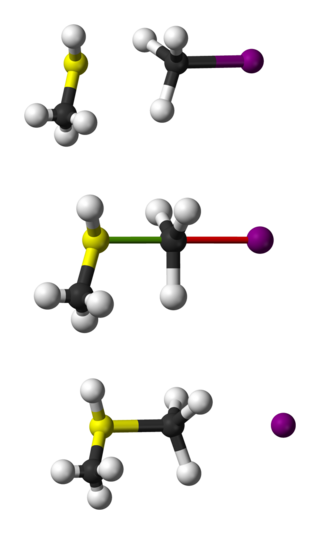
Bimolecular nucleophilic substitution (SN2) is a type of reaction mechanism that is common in organic chemistry. In the SN2 reaction, a strong nucleophile forms a new bond to an sp3-hybridised carbon atom via a backside attack, all while the leaving group detaches from the reaction center in a concerted fashion.
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound with formula CH2N4, of which three isomers can be formulated.
A superbase is a compound that has a particularly high affinity for protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.

Borazine, also known as borazole, is an inorganic compound with the chemical formula B3H6N3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. For this reason borazine is sometimes referred to as “inorganic benzene”. Like benzene, borazine is a colourless liquid with an aromatic odor.

Matrix isolation is an experimental technique used in chemistry and physics. It generally involves a material being trapped within an unreactive matrix. A host matrix is a continuous solid phase in which guest particles are embedded. The guest is said to be isolated within the host matrix. Initially the term matrix-isolation was used to describe the placing of a chemical species in any unreactive material, often polymers or resins, but more recently has referred specifically to gases in low-temperature solids. A typical matrix isolation experiment involves a guest sample being diluted in the gas phase with the host material, usually a noble gas or nitrogen. This mixture is then deposited on a window that is cooled to below the melting point of the host gas. The sample may then be studied using various spectroscopic procedures.
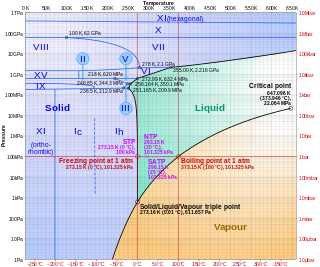
Amorphous ice is an amorphous solid form of water. Common ice is a crystalline material wherein the molecules are regularly arranged in a hexagonal lattice, whereas amorphous ice lacks long-range order in its molecular arrangement. Amorphous ice is produced either by rapid cooling of liquid water, or by compressing ordinary ice at low temperatures.

Polyamorphism is the ability of a substance to exist in several different amorphous modifications. It is analogous to the polymorphism of crystalline materials. Many amorphous substances can exist with different amorphous characteristics. However, polyamorphism requires two distinct amorphous states with a clear, discontinuous (first-order) phase transition between them. When such a transition occurs between two stable liquid states, a polyamorphic transition may also be referred to as a liquid–liquid phase transition.
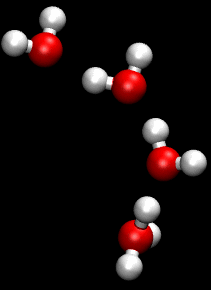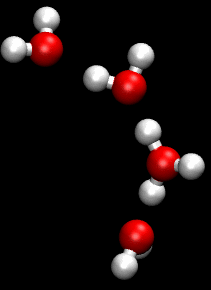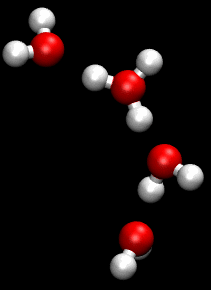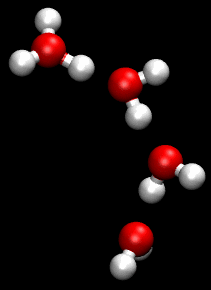
The Grotthuss mechanism is a model for the process by which an 'excess' proton or proton defect diffuses through the hydrogen bond network of water molecules or other hydrogen-bonded liquids through the formation and concomitant cleavage of covalent bonds involving neighboring molecules.
Tin(II) bromide is a chemical compound of tin and bromine with a chemical formula of SnBr2. Tin is in the +2 oxidation state. The stability of tin compounds in this oxidation state is attributed to the inert pair effect.

Carbon trioxide (CO3) is an unstable oxide of carbon (an oxocarbon). The possible isomers of carbon trioxide include ones with molecular symmetry point groups Cs, D3h, and C2v. The C2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. Carbon trioxide should not be confused with the stable carbonate ion (CO2−
3).
Ice Ic is a metastable cubic crystalline variant of ice. Hans König was the first to identify and deduce the structure of ice Ic. The oxygen atoms in ice Ic are arranged in a diamond structure and is extremely similar to ice Ih having nearly identical densities and the same lattice constant along the hexagonal puckered-planes. It forms at temperatures between 130 and 220 kelvins upon cooling, and can exist up to 240 K (−33 °C) upon warming, when it transforms into ice Ih.

Boron can be prepared in several crystalline and amorphous forms. Well known crystalline forms are α-rhombohedral (α-R), β-rhombohedral (β-R), and β-tetragonal (β-T). In special circumstances, boron can also be synthesized in the form of its α-tetragonal (α-T) and γ-orthorhombic (γ) allotropes. Two amorphous forms, one a finely divided powder and the other a glassy solid, are also known. Although at least 14 more allotropes have been reported, these other forms are based on tenuous evidence or have not been experimentally confirmed, or are thought to represent mixed allotropes, or boron frameworks stabilized by impurities. Whereas the β-rhombohedral phase is the most stable and the others are metastable, the transformation rate is negligible at room temperature, and thus all five phases can exist at ambient conditions. Amorphous powder boron and polycrystalline β-rhombohedral boron are the most common forms. The latter allotrope is a very hard grey material, about ten percent lighter than aluminium and with a melting point (2080 °C) several hundred degrees higher than that of steel.

Carbon nanotube chemistry involves chemical reactions, which are used to modify the properties of carbon nanotubes (CNTs). CNTs can be functionalized to attain desired properties that can be used in a wide variety of applications. The two main methods of CNT functionalization are covalent and non-covalent modifications.
1,1-Dihydroxyethene is an organic compound consisting of two hydroxy groups as substituents on the same carbon atom of an ethene chain. The chemical is also called ketene hydrate because it is the carbonyl hydrate of ketene. Its structure can also be considered as the enol form of acetic acid. This compound is likely a key intermediate in the hydration reaction that converts ketene into acetic acid. The analysis of the possible pathways for this reaction has been cited as an example of the importance of considering activation energy of each mechanistic step. The hydration of the carbonyl of ketene, which formally involves an addition reaction of one water molecule onto the carbonyl group, is likely catalyzed by a second water molecule. The compound has now been synthesized and identified spectroscopically.
Ice XIX is a proposed crystalline phase of water. Along with ice XV, it is one of two phases of ice directly related to ice VI. Ice XIX is prepared by cooling HCl-doped ice VI at a pressure above 1.6 GPa down to about 100 K. As of 2022, its crystal structure has not been elucidated.
Ice IV is a metastable high-pressure phase of ice. It is formed when liquid water is compressed with an immense force.