Venom or zootoxin is a type of toxin produced by an animal that is actively delivered through a wound by means of a bite, sting, or similar action. The toxin is delivered through a specially evolved venom apparatus, such as fangs or a stinger, in a process called envenomation. Venom is often distinguished from poison, which is a toxin that is passively delivered by being ingested, inhaled, or absorbed through the skin, and toxungen, which is actively transferred to the external surface of another animal via a physical delivery mechanism.

Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral nervous system, muscle, and many other tissues of many organisms. At the neuromuscular junction they are the primary receptor in muscle for motor nerve-muscle communication that controls muscle contraction. In the peripheral nervous system: (1) they transmit outgoing signals from the presynaptic to the postsynaptic cells within the sympathetic and parasympathetic nervous system, and (2) they are the receptors found on skeletal muscle that receive acetylcholine released to signal for muscular contraction. In the immune system, nAChRs regulate inflammatory processes and signal through distinct intracellular pathways. In insects, the cholinergic system is limited to the central nervous system.

Snake venom is a highly toxic saliva containing zootoxins that facilitates in the immobilization and digestion of prey. This also provides defense against threats. Snake venom is injected by unique fangs during a bite, whereas some species are also able to spit venom.
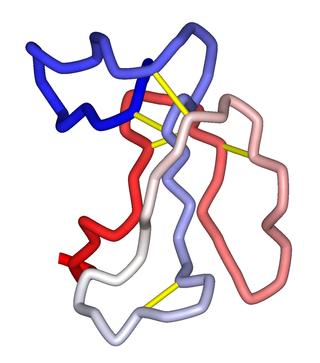
α-Bungarotoxin is one of the bungarotoxins, components of the venom of the elapid Taiwanese banded krait snake. It is a type of α-neurotoxin, a neurotoxic protein that is known to bind competitively and in a relatively irreversible manner to the nicotinic acetylcholine receptor found at the neuromuscular junction, causing paralysis, respiratory failure, and death in the victim. It has also been shown to play an antagonistic role in the binding of the α7 nicotinic acetylcholine receptor in the brain, and as such has numerous applications in neuroscience research.

Calciseptine (CaS) is a natural neurotoxin isolated from the black mamba Dendroaspis p. polylepis venom. This toxin consists of 60 amino acids with four disulfide bonds. Calciseptine specifically blocks L-type calcium channels, but not other voltage-dependent Ca2+ channels such as N-type and T-type channels.
Taipoxin is a potent myo- and neurotoxin that was isolated from the venom of the coastal taipan Oxyuranus scutellatus or also known as the common taipan. Taipoxin like many other pre-synaptic neurotoxins are phospholipase A2 (PLA2) toxins, which inhibit/complete block the release of the motor transmitter acetylcholine and lead to death by paralysis of the respiratory muscles (asphyxia). It is the most lethal neurotoxin isolated from any snake venom to date.
Bungarotoxins are toxins found in the venom of snakes and kraits. Bites from these animals can result in severe symptoms including bleeding or hemorrhage, paralysis and tissue damage that can result in amputation. The paralytic effects of venom are particularly dangerous as they can impair breathing. These symptoms are the result of bungarotoxin presence in the venom. In actuality, venom contains several distinct bungarotoxins, each varying in which receptors they act on and how powerful they are.

α-Cobratoxin is a substance of the venom of certain Naja cobras. It is a nicotinic acetylcholine receptor (nAChR) antagonist which causes paralysis by preventing the binding of acetylcholine to the nAChR.

The high-affinity choline transporter (ChT) also known as solute carrier family 5 member 7 is a protein in humans that is encoded by the SLC5A7 gene. It is a cell membrane transporter and carries choline into acetylcholine-synthesizing neurons.

The LU domain is an evolutionarily conserved protein domain of the three-finger protein superfamily. This domain is found in the extracellular domains of cell-surface receptors and in either GPI-anchored or secreted globular proteins, for example the Ly-6 family, CD59, and Sgp-2.

α-Neurotoxins are a group of neurotoxic peptides found in the venom of snakes in the families Elapidae and Hydrophiidae. They can cause paralysis, respiratory failure, and death. Members of the three-finger toxin protein family, they are antagonists of post-synaptic nicotinic acetylcholine receptors (nAChRs) in the neuromuscular synapse that bind competitively and irreversibly, preventing synaptic acetylcholine (ACh) from opening the ion channel. Over 100 α-neurotoxins have been identified and sequenced.

Mambalgins are peptides found in the venom of the black mamba, an elapid snake. Mambalgins are members of the three-finger toxin (3FTx) protein family and have the characteristic three-finger protein fold. First reported by French researchers in 2012, mambalgins are unusual members of the 3FTx family in that they have the in vivo effect of causing analgesia without apparent toxicity. Their mechanism of action is potent inhibition of acid-sensing ion channels.

Venom in snakes and some lizards is a form of saliva that has been modified into venom over its evolutionary history. In snakes, venom has evolved to kill or subdue prey, as well as to perform other diet-related functions. While snakes occasionally use their venom in self defense, this is not believed to have had a strong effect on venom evolution. The evolution of venom is thought to be responsible for the enormous expansion of snakes across the globe.

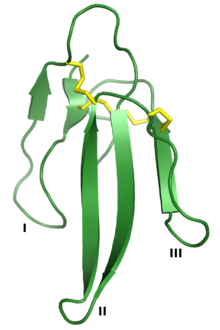
Three-finger toxins are a protein superfamily of small toxin proteins found in the venom of snakes. Three-finger toxins are in turn members of a larger superfamily of three-finger protein domains which includes non-toxic proteins that share a similar protein fold. The group is named for its common structure consisting of three beta strand loops connected to a central core containing four conserved disulfide bonds. The 3FP protein domain has no enzymatic activity and is typically between 60-74 amino acid residues long. Despite their conserved structure, three-finger toxin proteins have a wide range of pharmacological effects. Most members of the family are neurotoxins that act on cholinergic intercellular signaling; the alpha-neurotoxin family interacts with muscle nicotinic acetylcholine receptors (nAChRs), the kappa-bungarotoxin family with neuronal nAChRs, and muscarinic toxins with muscarinic acetylcholine receptors (mAChRs).

Irditoxin is a three-finger toxin (3FTx) protein found in the venom of the brown tree snake and likely in other members of the genus Boiga. It is a heterodimer composed of two distinct protein chains, each of the three-finger protein fold, linked by an intermolecular disulfide bond. This structure is unusual for 3FTx proteins, which are most commonly monomeric.
Mipartoxin-I is a neurotoxin produced by Micrurus mipartitus, a venomous coral snake distributed in Central and South America. This toxin causes a neuromuscular blockade by blocking the nicotinic acetylcholine receptor. It is the most abundant component in the venom.
Long neurotoxin 1 (LNTX-1) is a neurotoxin that binds antagonistically to all types of muscular and neuronal nicotinic acetylcholine receptors. LNTX-1 is found in the venom of the king cobra.
Crotoxin (CTX) is the main toxic compound in the snake venom of the South American rattlesnake, Crotalus durissus terrificus. Crotoxin is a heterodimeric beta-neurotoxin, composed of an acidic, non-toxic and non-enzymatic subunit (CA), and a basic, weakly toxic, phospholipase A2 protein (CB). This neurotoxin causes paralysis by both pre- and postsynaptic blocking of acetylcholine signalling.

LY6/PLAUR Domain Containing 6B, also known under the name Cancer/Testis Antigen 116 (CTA116) and LYPD7 is encoded by the LYPD6B gene. LYPD6B is a member of the lymphocyte antigen 6 (LY6) protein family. It is expressed in the testis, lungs, stomach, prostate and in the nervous system where it acts as a modulator of nicotinic acetylcholine receptor (nAChRs) activity.
Venomics is the large-scale study of proteins associated with venom. Venom is a toxic substance secreted by animals, which is typically injected either offensively or defensively into prey or aggressors, respectively.