
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine.
A monolayer is a single, closely packed layer of entities, commonly atoms or molecules. Monolayers can also be made out of cells. Self-assembled monolayers form spontaneously on surfaces. Monolayers of layered crystals like graphene and molybdenum disulfide are generally called 2D materials.

Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid is dissolved by or permeates a liquid or solid. While adsorption does often precede absorption, which involves the transfer of the absorbate into the volume of the absorbent material, alternatively, adsorption is distinctly a surface phenomenon, wherein the adsorbate does not penetrate through the material surface and into the bulk of the adsorbent. The term sorption encompasses both adsorption and absorption, and desorption is the reverse of sorption.

Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with the first one. The degree of wetting (wettability) is determined by a force balance between adhesive and cohesive forces. There are two types of wetting: non-reactive wetting and reactive wetting.

An implant is a medical device manufactured to replace a missing biological structure, support a damaged biological structure, or enhance an existing biological structure. For example, an implant may be a rod, used to strengthen weak bones. Medical implants are human-made devices, in contrast to a transplant, which is a transplanted biomedical tissue. The surface of implants that contact the body might be made of a biomedical material such as titanium, silicone, or apatite depending on what is the most functional. In 2018, for example, American Elements developed a nickel alloy powder for 3D printing robust, long-lasting, and biocompatible medical implants. In some cases implants contain electronics, e.g. artificial pacemaker and cochlear implants. Some implants are bioactive, such as subcutaneous drug delivery devices in the form of implantable pills or drug-eluting stents.
Titanium aluminide, commonly gamma titanium, is an intermetallic chemical compound. It is lightweight and resistant to oxidation and heat, but has low ductility. The density of γ-TiAl is about 4.0 g/cm3. It finds use in several applications including aircraft, jet engines, sporting equipment and automobiles. The development of TiAl based alloys began circa 1970. The alloys have been used in these applications only since about 2000.

A foreign body reaction (FBR) is a typical tissue response to a foreign body within biological tissue. It usually includes the formation of a foreign body granuloma. Tissue-encapsulation of an implant is an example, as is inflammation around a splinter. Foreign body granuloma formation consists of protein adsorption, macrophages, multinucleated foreign body giant cells, fibroblasts, and angiogenesis. It has also been proposed that the mechanical property of the interface between an implant and its surrounding tissues is critical for the host response.
Nitinol biocompatibility is an important factor in biomedical applications. Nitinol (NiTi), which is formed by alloying nickel and titanium, is a shape-memory alloy with superelastic properties more similar to that of bone, when compared to stainless steel, another commonly used biomaterial. Biomedical applications that utilize nitinol include stents, heart valve tools, bone anchors, staples, septal defect devices and implants. It is a commonly used biomaterial especially in the development of stent technology.

Bioceramics and bioglasses are ceramic materials that are biocompatible. Bioceramics are an important subset of biomaterials. Bioceramics range in biocompatibility from the ceramic oxides, which are inert in the body, to the other extreme of resorbable materials, which are eventually replaced by the body after they have assisted repair. Bioceramics are used in many types of medical procedures. Bioceramics are typically used as rigid materials in surgical implants, though some bioceramics are flexible. The ceramic materials used are not the same as porcelain type ceramic materials. Rather, bioceramics are closely related to either the body's own materials or are extremely durable metal oxides.
Adsorption is the accumulation and adhesion of molecules, atoms, ions, or larger particles to a surface, but without surface penetration occurring. The adsorption of larger biomolecules such as proteins is of high physiological relevance, and as such they adsorb with different mechanisms than their molecular or atomic analogs. Some of the major driving forces behind protein adsorption include: surface energy, intermolecular forces, hydrophobicity, and ionic or electrostatic interaction. By knowing how these factors affect protein adsorption, they can then be manipulated by machining, alloying, and other engineering techniques to select for the most optimal performance in biomedical or physiological applications.
Adsorption is the adhesion of ions or molecules onto the surface of another phase. Adsorption may occur via physisorption and chemisorption. Ions and molecules can adsorb to many types of surfaces including polymer surfaces. A polymer is a large molecule composed of repeating subunits bound together by covalent bonds. In dilute solution, polymers form globule structures. When a polymer adsorbs to a surface that it interacts favorably with, the globule is essentially squashed, and the polymer has a pancake structure.

Biomaterials are materials that are used in contact with biological systems. Biocompatibility and applicability of surface modification with current uses of metallic, polymeric and ceramic biomaterials allow alteration of properties to enhance performance in a biological environment while retaining bulk properties of the desired device.
Protein adsorption refers to the adhesion of proteins to solid surfaces. This phenomenon is an important issue in the food processing industry, particularly in milk processing and wine and beer making. Excessive adsorption, or protein fouling, can lead to health and sanitation issues, as the adsorbed protein is very difficult to clean and can harbor bacteria, as is the case in biofilms. Product quality can be adversely affected if the adsorbed material interferes with processing steps, like pasteurization. However, in some cases protein adsorption is used to improve food quality, as is the case in fining of wines.
The strength of metal oxide adhesion effectively determines the wetting of the metal-oxide interface. The strength of this adhesion is important, for instance, in production of light bulbs and fiber-matrix composites that depend on the optimization of wetting to create metal-ceramic interfaces. The strength of adhesion also determines the extent of dispersion on catalytically active metal. Metal oxide adhesion is important for applications such as complementary metal oxide semiconductor devices. These devices make possible the high packing densities of modern integrated circuits.
Ti-6Al-4V, also sometimes called TC4, Ti64, or ASTM Grade 5, is an alpha-beta titanium alloy with a high specific strength and excellent corrosion resistance. It is one of the most commonly used titanium alloys and is applied in a wide range of applications where low density and excellent corrosion resistance are necessary such as e.g. aerospace industry and biomechanical applications.
Biomaterials exhibit various degrees of compatibility with the harsh environment within a living organism. They need to be nonreactive chemically and physically with the body, as well as integrate when deposited into tissue. The extent of compatibility varies based on the application and material required. Often modifications to the surface of a biomaterial system are required to maximize performance. The surface can be modified in many ways, including plasma modification and applying coatings to the substrate. Surface modifications can be used to affect surface energy, adhesion, biocompatibility, chemical inertness, lubricity, sterility, asepsis, thrombogenicity, susceptibility to corrosion, degradation, and hydrophilicity.
Ultra-low fouling is a rating of a surface's ability to shed potential contamination. Surfaces are prone to contamination, which is a phenomenon known as fouling. Unwanted adsorbates caused by fouling change the properties of a surface, which is often counter-productive to the function of that surface. Consequently, a necessity for anti-fouling surfaces has arisen in many fields: blocked pipes inhibit factory productivity, biofouling increases fuel consumption on ships, medical devices must be kept sanitary, etc. Although chemical fouling inhibitors, metallic coatings, and cleaning processes can be used to reduce fouling, non-toxic surfaces with anti-fouling properties are ideal for fouling prevention. To be considered effective, an ultra-low fouling surface must be able to repel and withstand the accumulation of detrimental aggregates down to less than 5 ng/cm2. A recent surge of research has been conducted to create these surfaces in order to benefit the biological, nautical, mechanical, and medical fields.
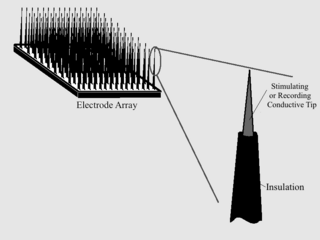
As with any material implanted in the body, it is important to minimize or eliminate foreign body response and maximize effectual integration. Neural implants have the potential to increase the quality of life for patients with such disabilities as Alzheimer's, Parkinson's, epilepsy, depression, and migraines. With the complexity of interfaces between a neural implant and brain tissue, adverse reactions such as fibrous tissue encapsulation that hinder the functionality, occur. Surface modifications to these implants can help improve the tissue-implant interface, increasing the lifetime and effectiveness of the implant.
Ti-6Al-7Nb is an alpha-beta titanium alloy first synthesized in 1977 containing 6% aluminum and 7% niobium. It features high strength and has similar properties as the cytotoxic vanadium containing alloy Ti-6Al-4V. Ti-6Al-7Nb is used as a material for hip prostheses.
Titanium foams exhibit high specific strength, high energy absorption, excellent corrosion resistance and biocompatibility. These materials are ideally suited for applications within the aerospace industry. An inherent resistance to corrosion allows the foam to be a desirable candidate for various filtering applications. Further, titanium's physiological inertness makes its porous form a promising candidate for biomedical implantation devices. The largest advantage in fabricating titanium foams is that the mechanical and functional properties can be adjusted through manufacturing manipulations that vary porosity and cell morphology. The high appeal of titanium foams is directly correlated to a multi-industry demand for advancement in this technology.
















