Cancer immunotherapy is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.

c-Met, also called tyrosine-protein kinase Met or hepatocyte growth factor receptor (HGFR), is a protein that in humans is encoded by the MET gene. The protein possesses tyrosine kinase activity. The primary single chain precursor protein is post-translationally cleaved to produce the alpha and beta subunits, which are disulfide linked to form the mature receptor.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.
Panitumumab, sold under the brand name Vectibix, is a fully human monoclonal antibody specific to the epidermal growth factor receptor.
Bavituximab (PGN401) is a human-mouse chimeric monoclonal antibody against phosphatidylserine, which is a component of cell membranes that is exposed when a cell is transformed into solid tumor cancer cell or dies, and when cells are infected with hepatitis C. The process of cell death is highly controlled and so there usually no immune response to phosphatidylserine but when bavituximab binds to it, the conjugate appears to stimulate an immune response in humans.
Tremelimumab, sold under the brand name Imjudo, is a fully human monoclonal antibody against CTLA-4. It is an immune checkpoint blocker.
Matuzumab is a humanized monoclonal antibody for the treatment of cancer. It binds to the epidermal growth factor receptor (EGFR) with high affinity. The mouse monoclonal antibody (mAb425) from which matuzumab was developed at the Wistar Institute in Philadelphia, Pennsylvania
Nimotuzumab is a humanized monoclonal antibody that as of 2014 had orphan status in the US and EU for glioma, and marketing approval in India, China, and other countries for squamous cell carcinomas of the head and neck, and was undergoing several clinical trials.
Zalutumumab is a fully human IgG1 monoclonal antibody (mAb) directed towards the epidermal growth factor receptor (EGFR). It is a product developed by Genmab in Utrecht, the Netherlands. Specifically, zalutumumab is designed for the treatment of squamous cell carcinoma of the head and neck (SCCHN), a type of cancer.
Axitinib, sold under the brand name Inlyta, is a small molecule tyrosine kinase inhibitor developed by Pfizer. It has been shown to significantly inhibit growth of breast cancer in animal (xenograft) models and has shown partial responses in clinical trials with renal cell carcinoma (RCC) and several other tumour types.
Cixutumumab (IMC-A12) is a human monoclonal antibody for the treatment of solid tumors.

Lenvatinib, sold under the brand name Lenvima among others, is an anti-cancer medication for the treatment of certain kinds of thyroid cancer and for other cancers as well. It was developed by Eisai Co. and acts as a multiple kinase inhibitor against the VEGFR1, VEGFR2 and VEGFR3 kinases.
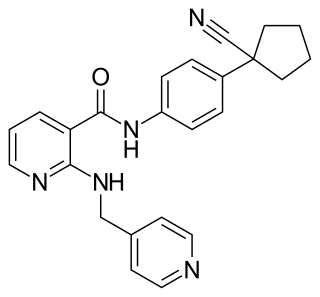
Apatinib, also known as rivoceranib, is a tyrosine kinase inhibitor that selectively inhibits the vascular endothelial growth factor receptor-2. It is an orally bioavailable, small molecule agent which is thought to inhibit angiogenesis in cancer cells; specifically, apatinib inhibits VEGF-mediated endothelial cell migration and proliferation thus blocking new blood vessel formation in tumor tissue. This agent also mildly inhibits c-Kit and c-SRC tyrosine kinases.

Zoptarelin doxorubicin consists of doxorubicin linked to a small peptide agonist to the luteinizing hormone-releasing hormone (LHRH) receptor. It has been developed as a potential treatment for a number of human cancers. The LHRH receptor is aberrantly present on the cell surface of approximately 80% of endometrial and ovarian cancers, 86% of prostate cancers and about 50% of breast cancers. Whereas in normal tissues, expression of this receptor is mainly confined to the pituitary gland, reproductive organs and hematopoietic stem cells. To a lesser extent the LHRH receptor is also found on the surface of bladder, colorectal, and pancreatic cancers, sarcomas, lymphomas, melanomas, and renal cell carcinomas.

Cabozantinib, sold under the brand names Cometriq and Cabometyx among others, is a medication used to treat medullary thyroid cancer, renal cell carcinoma, and hepatocellular carcinoma. It is a small molecule inhibitor of the tyrosine kinases c-Met and VEGFR2, and also inhibits AXL and RET. It was discovered and developed by Exelixis Inc.
JX-594 is an oncolytic virus is designed to target and destroy cancer cells. It is also known as Pexa-Vec, INN pexastimogene devacirepvec) and was constructed in Dr. Edmund Lattime's lab at Thomas Jefferson University, tested in clinical trials on melanoma patients, and licensed and further developed by SillaJen.
c-Met inhibitors are a class of small molecules that inhibit the enzymatic activity of the c-Met tyrosine kinase, the receptor of hepatocyte growth factor/scatter factor (HGF/SF). These inhibitors may have therapeutic application in the treatment of various types of cancers.

Brivanib alaninate (INN/USAN) also known as BMS-582664 is an investigational, anti-tumorigenic drug for oral administration. The drug is being developed by Bristol-Myers Squibb for the treatment of hepatocellular carcinoma or HCC, the most common type of liver cancer. Brivanib is no longer in active development.
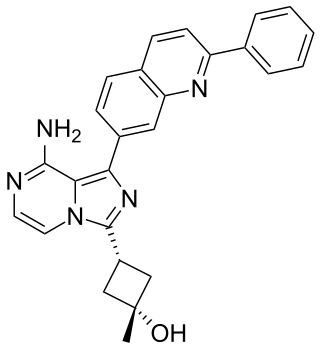
Linsitinib is an experimental drug candidate for the treatment of various types of cancer. It is an inhibitor of the insulin receptor and of the insulin-like growth factor 1 receptor (IGF-1R). This prevents tumor cell proliferation and induces tumor cell apoptosis.
Tumor-associated macrophages (TAMs) are a class of immune cells present in high numbers in the microenvironment of solid tumors. They are heavily involved in cancer-related inflammation. Macrophages are known to originate from bone marrow-derived blood monocytes or yolk sac progenitors, but the exact origin of TAMs in human tumors remains to be elucidated. The composition of monocyte-derived macrophages and tissue-resident macrophages in the tumor microenvironment depends on the tumor type, stage, size, and location, thus it has been proposed that TAM identity and heterogeneity is the outcome of interactions between tumor-derived, tissue-specific, and developmental signals.