
Berkelium is a synthetic chemical element; it has symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium.

Benzoic acid is a white solid organic compound with the formula C6H5COOH, whose structure consists of a benzene ring with a carboxyl substituent. The benzoyl group is often abbreviated "Bz", thus benzoic acid is also denoted as BzOH, since the benzoyl group has the formula –C6H5CO. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source.

Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at 114 °C (237 °F), and boils to a violet gas at 184 °C (363 °F). The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek Ιώδης 'violet-coloured'.

Rhodium is a chemical element; it has symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isotope, which is 103Rh. Naturally occurring rhodium is usually found as a free metal or as an alloy with similar metals and rarely as a chemical compound in minerals such as bowieite and rhodplumsite. It is one of the rarest and most valuable precious metals.

The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans, it is in the neck and consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the isthmus (pl.: isthmi). The thyroid gland is a butterfly-shaped gland located in the neck below the Adam's apple. Microscopically, the functional unit of the thyroid gland is the spherical thyroid follicle, lined with follicular cells (thyrocytes), and occasional parafollicular cells that surround a lumen containing colloid. The thyroid gland secretes three hormones: the two thyroid hormones – triiodothyronine (T3) and thyroxine (T4) – and a peptide hormone, calcitonin. The thyroid hormones influence the metabolic rate and protein synthesis and growth and development in children. Calcitonin plays a role in calcium homeostasis. Secretion of the two thyroid hormones is regulated by thyroid-stimulating hormone (TSH), which is secreted from the anterior pituitary gland. TSH is regulated by thyrotropin-releasing hormone (TRH), which is produced by the hypothalamus.

Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.

A perchlorate is a chemical compound containing the perchlorate ion, ClO4−, the conjugate base of perchloric acid (ionic perchlorate). As counterions, there can be metal cations, quaternary ammonium cations or other ions, for example, nitronium cation (NO2+).

Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy, and gamma rays. The two smaller nuclei are the fission products..

Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2. It forms hydrates. Zinc chloride, anhydrous and its hydrates are colorless or white crystalline solids, and are highly soluble in water. Five hydrates of zinc chloride are known, as well as four forms of anhydrous zinc chloride. This salt is hygroscopic and even deliquescent. Zinc chloride finds wide application in textile processing, metallurgical fluxes, and chemical synthesis. No mineral with this chemical composition is known aside from the very rare mineral simonkolleite, Zn5(OH)8Cl2·H2O.

Potassium iodide is a chemical compound, medication, and dietary supplement. It is a medication used for treating hyperthyroidism, in radiation emergencies, and for protecting the thyroid gland when certain types of radiopharmaceuticals are used. In the third world it is also used for treating skin sporotrichosis and phycomycosis. It is a supplement used by people with low dietary intake of iodine. It is administered orally.

Potassium perchlorate is the inorganic salt with the chemical formula KClO4. Like other perchlorates, this salt is a strong oxidizer although it usually reacts very slowly with organic substances. This, usually obtained as a colorless, crystalline solid, is a common oxidizer used in fireworks, ammunition percussion caps, explosive primers, and is used variously in propellants, flash compositions, stars, and sparklers. It has been used as a solid rocket propellant, although in that application it has mostly been replaced by the higher performance ammonium perchlorate.

Levothyroxine, also known as L-thyroxine, is a synthetic form of the thyroid hormone thyroxine (T4). It is used to treat thyroid hormone deficiency (hypothyroidism), including a severe form known as myxedema coma. It may also be used to treat and prevent certain types of thyroid tumors. It is not indicated for weight loss. Levothyroxine is taken orally (by mouth) or given by intravenous injection. Levothyroxine has a half-life of 7.5 days when taken daily, so about six weeks is required for it to reach a steady level in the blood.

Thionyl chloride is an inorganic compound with the chemical formula SOCl2. It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.

Iodine-131 is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because 131I is a major fission product of uranium and plutonium, comprising nearly 3% of the total products of fission. See fission product yield for a comparison with other radioactive fission products. 131I is also a major fission product of uranium-233, produced from thorium.
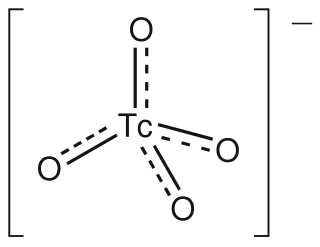
The pertechnetate ion is an oxyanion with the chemical formula TcO−
4. It is often used as a convenient water-soluble source of isotopes of the radioactive element technetium (Tc). In particular it is used to carry the 99mTc isotope which is commonly used in nuclear medicine in several nuclear scanning procedures.

There are 37 known isotopes of iodine (53I) from 108I to 144I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element.

Benzotrichloride (BTC), also known as α,α,α-trichlorotoluene, phenyl chloroform or (trichloromethyl)benzene, is an organic compound with the formula C6H5CCl3. Benzotrichloride is an unstable, colorless or somewhat yellowish, viscous, chlorinated hydrocarbon with a penetrating odor. Benzotrichloride is used extensively as a chemical intermediate for products of various classes, i.e. dyes and antimicrobial agents.
Iodine is an essential trace element in biological systems. It has the distinction of being the heaviest element commonly needed by living organisms as well as the second-heaviest known to be used by any form of life. It is a component of biochemical pathways in organisms from all biological kingdoms, suggesting its fundamental significance throughout the evolutionary history of life.
Dimethyl tetrachloroterephthalate (DCPA, with the main trade name Dacthal) is an organic compound with the formula C6Cl4(CO2CH3)2. It is the dimethyl ester of tetrachloroterephthalic acid, used as a preemergent herbicide with the ISO common name chlorthal-dimethyl. It kills annual grasses and many common weeds without killing sensitive plants such as turf grasses, flowers, fruits, vegetables, and cotton.

Prothioconazole is a synthetic chemical produced primarily for its fungicidal properties. It is a member of the class of compounds triazoles, and possesses a unique toxophore in this class of fungicides. Its effective fungicidal properties can be attributed to its ability to inhibit CYP51A1. This enzyme is required to biosynthesize ergosterol, a key component in the cell membrane of fungi.



















