Related Research Articles

Pneumonia is an inflammatory condition of the lung primarily affecting the small air sacs known as alveoli. Symptoms typically include some combination of productive or dry cough, chest pain, fever, and difficulty breathing. The severity of the condition is variable.

The common cold or the cold is a viral infectious disease of the upper respiratory tract that primarily affects the respiratory mucosa of the nose, throat, sinuses, and larynx. Signs and symptoms may appear fewer than two days after exposure to the virus. These may include coughing, sore throat, runny nose, sneezing, headache, and fever. People usually recover in seven to ten days, but some symptoms may last up to three weeks. Occasionally, those with other health problems may develop pneumonia.

Cochrane is a British international charitable organisation formed to organise medical research findings to facilitate evidence-based choices about health interventions involving health professionals, patients and policy makers. It includes 53 review groups that are based at research institutions worldwide. Cochrane has approximately 30,000 volunteer experts from around the world.

Croup, also known as laryngotracheobronchitis, is a type of respiratory infection that is usually caused by a virus. The infection leads to swelling inside the trachea, which interferes with normal breathing and produces the classic symptoms of "barking/brassy" cough, inspiratory stridor and a hoarse voice. Fever and runny nose may also be present. These symptoms may be mild, moderate, or severe. Often it starts or is worse at night and normally lasts one to two days.
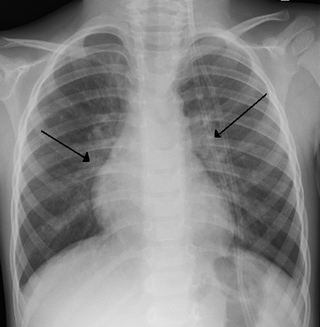
Bronchiolitis is inflammation of the small airways in the lungs. Acute bronchiolitis is due to a viral infection usually affecting children younger than two years of age. Symptoms may include fever, cough, runny nose, wheezing, and breathing problems. More severe cases may be associated with nasal flaring, grunting, or the skin between the ribs pulling in with breathing. If the child has not been able to feed properly, signs of dehydration may be present.

An upper respiratory tract infection (URTI) is an illness caused by an acute infection, which involves the upper respiratory tract, including the nose, sinuses, pharynx, larynx or trachea. This commonly includes nasal obstruction, sore throat, tonsillitis, pharyngitis, laryngitis, sinusitis, otitis media, and the common cold. Most infections are viral in nature, and in other instances, the cause is bacterial. URTIs can also be fungal or helminthic in origin, but these are less common.

Zanamivir is a medication used to treat and prevent influenza caused by influenza A and influenza B viruses. It is a neuraminidase inhibitor and was developed by the Australian biotech firm Biota Holdings. It was licensed to Glaxo in 1990 and approved in the US in 1999, only for use as a treatment for influenza. In 2006, it was approved for prevention of influenza A and B. Zanamivir was the first neuraminidase inhibitor commercially developed. It is marketed by GlaxoSmithKline under the trade name Relenza as a powder for oral inhalation.
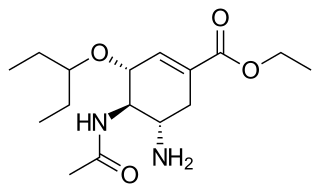
Oseltamivir, sold under the brand name Tamiflu, is an antiviral medication used to treat and prevent influenza A and influenza B, viruses that cause the flu. Many medical organizations recommend it in people who have complications or are at high risk of complications within 48 hours of first symptoms of infection. They recommend it to prevent infection in those at high risk, but not the general population. The Centers for Disease Control and Prevention (CDC) recommends that clinicians use their discretion to treat those at lower risk who present within 48 hours of first symptoms of infection. It is taken by mouth, either as a pill or liquid.

Lower respiratory tract infection (LRTI) is a term often used as a synonym for pneumonia but can also be applied to other types of infection including lung abscess and acute bronchitis. Symptoms include shortness of breath, weakness, fever, coughing and fatigue. A routine chest X-ray is not always necessary for people who have symptoms of a lower respiratory tract infection.

Influenza vaccines, also known as flu shots, are vaccines that protect against infection by influenza viruses. New versions of the vaccines are developed twice a year, as the influenza virus rapidly changes. While their effectiveness varies from year to year, most provide modest to high protection against influenza. The United States Centers for Disease Control and Prevention (CDC) estimates that vaccination against influenza reduces sickness, medical visits, hospitalizations, and deaths. Immunized workers who do catch the flu return to work half a day sooner on average. Vaccine effectiveness in those over 65 years old remains uncertain due to a lack of high-quality research.
Neuraminidase inhibitors (NAIs) are a class of drugs which block the neuraminidase enzyme. They are a commonly used antiviral drug type against influenza. Viral neuraminidases are essential for influenza reproduction, facilitating viral budding from the host cell. Oseltamivir (Tamiflu), zanamivir (Relenza), laninamivir (Inavir), and peramivir belong to this class. Unlike the M2 inhibitors, which work only against the influenza A virus, NAIs act against both influenza A and influenza B.

Respiratory tract infections (RTIs) are infectious diseases involving the respiratory tract. An infection of this type usually is further classified as an upper respiratory tract infection or a lower respiratory tract infection. Lower respiratory infections, such as pneumonia, tend to be far more severe than upper respiratory infections, such as the common cold.
Immunization during pregnancy is the administration of a vaccine to a pregnant individual. This may be done either to protect the individual from disease or to induce an antibody response, such that the antibodies cross the placenta and provide passive immunity to the infant after birth. In many countries, including the US, Canada, UK, Australia and New Zealand, vaccination against influenza, COVID-19 and whooping cough is routinely offered during pregnancy.

Influenza, commonly known as "the flu", is an infectious disease caused by influenza viruses. Symptoms range from mild to severe and often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. These symptoms begin from one to four days after exposure to the virus and last for about 2–8 days. Diarrhea and vomiting can occur, particularly in children. Influenza may progress to pneumonia, which can be caused by the virus or by a subsequent bacterial infection. Other complications of infection include acute respiratory distress syndrome, meningitis, encephalitis, and worsening of pre-existing health problems such as asthma and cardiovascular disease.
Peter Christian Gøtzsche is a Danish physician, medical researcher, and former leader of the Nordic Cochrane Center at Rigshospitalet in Copenhagen, Denmark. He is a co-founder of the Cochrane Collaboration and has written numerous reviews for the organization. His membership in Cochrane was terminated by its Governing Board of Trustees on 25 September 2018.
The Centre for Evidence-Based Medicine (CEBM), based in the Nuffield Department of Primary Care Health Sciences at the University of Oxford, is an academic-led centre dedicated to the practice, teaching, and dissemination of high quality evidence-based medicine to improve healthcare in everyday clinical practice. CEBM was founded by David Sackett in 1995. It was subsequently directed by Brian Haynes and Paul Glasziou. Since 2010 it has been led by Professor Carl Heneghan, a clinical epidemiologist and general practitioner.
Extensive investigation into vaccines and autism spectrum disorder has shown that there is no relationship between the two, causal or otherwise, and that the vaccine ingredients do not cause autism. Vaccinologist Peter Hotez researched the growth of the false claim and concluded that its spread originated with Andrew Wakefield's fraudulent 1998 paper, with no prior paper supporting a link.
Although several medications have been approved in different countries as of April 2022, not all countries have these medications. Patients with mild to moderate symptoms who are in the risk groups can take nirmatrelvir/ritonavir or remdesivir, either of which reduces the risk of serious illness or hospitalization. In the US, the Biden Administration COVID-19 action plan includes the Test to Treat initiative, where people can go to a pharmacy, take a COVID test, and immediately receive free Paxlovid if they test positive.
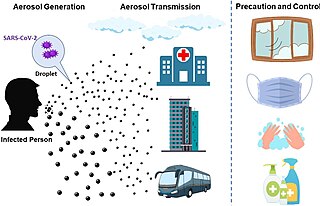
The transmission of COVID-19 is the passing of coronavirus disease 2019 from person to person. COVID-19 is mainly transmitted when people breathe in air contaminated by droplets/aerosols and small airborne particles containing the virus. Infected people exhale those particles as they breathe, talk, cough, sneeze, or sing. Transmission is more likely the closer people are. However, infection can occur over longer distances, particularly indoors.
Viral interference, also known as superinfection resistance, is the inhibition of viral reproduction caused by previous exposure of cells to another virus. The exact mechanism for viral interference is unknown. Factors that have been implicated are the generation of interferons by infected cells, and the occupation or down-modulation of cellular receptors.
References
- 1 2 "Dr. Tom Jefferson - Senior Clinical Tutor at University of Oxford". Tom Jefferson. Retrieved 2022-09-13.
- ↑ Brownlee, Shannon (1 November 2009). "Does the Vaccine Matter?". The Atlantic. Retrieved 8 December 2014.
- 1 2 "Spotlight on the Cochrane Acute Respiratory Infections Review Group". Cochrane Collaboration. 17 August 2011. Retrieved 8 December 2014.
- ↑ "History of Brighton Collaboration". Brighton Collaboration. Retrieved 6 July 2020.
- 1 2 3 4 5 6 Thomas, Katie (30 June 2013). "Breaking the Seal on Drug Research". New York Times. Retrieved 8 December 2014.
- ↑ Grush, Loren (18 January 2012). "Researchers claim inadequate research to support anti-influenza drug". Fox News. Retrieved 8 December 2014.
- ↑ Jefferson, T (2014-05-06), Jones MA, Doshi P, Del Mar CB, Hama R, Thompson MJ, Spencer EA, Onakpoya I, Mahtani KR, Nunan D, Howick J, Heneghan CJ, "Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children", Cochrane Database of Systematic Reviews, no. 4, doi:10.1002/14651858.CD008965.pub4, PMC 6464969 , CD008965, retrieved 2022-09-08– via The James Lind Library
- ↑ "CEBM blogs & media articles". dr. Tom Jefferson. Retrieved 2022-09-08.
- ↑ "Editorial and Grant Peer Review". Tom Jefferson. Retrieved 2022-06-18.
- ↑ Heneghan, Carl. "Trust the Evidence". Substack. Retrieved 2022-09-18.
- ↑ Onakpoya, Igho J.; Heneghan, Carl J.; Spencer, Elizabeth A.; Brassey, Jon; Rosca, Elena C.; Maltoni, Susanna; Plüddemann, Annette; Evans, David H.; Conly, John M.; Jefferson, Tom (2022-09-13). "Viral Cultures for Assessing Fomite Transmission of SARS-CoV-2: a Systematic Review and Meta-Analysis". Journal of Hospital Infection. 130: 63–94. doi:10.1016/j.jhin.2022.09.007. ISSN 0195-6701. PMC 9473144 . PMID 36115620.