Molecular biology is a branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions.
A restriction enzyme, restriction endonuclease, REase, ENase orrestrictase is an enzyme that cleaves DNA into fragments at or near specific recognition sites within molecules known as restriction sites. Restriction enzymes are one class of the broader endonuclease group of enzymes. Restriction enzymes are commonly classified into five types, which differ in their structure and whether they cut their DNA substrate at their recognition site, or if the recognition and cleavage sites are separate from one another. To cut DNA, all restriction enzymes make two incisions, once through each sugar-phosphate backbone of the DNA double helix.
In genetics, an expressed sequence tag (EST) is a short sub-sequence of a cDNA sequence. ESTs may be used to identify gene transcripts, and were instrumental in gene discovery and in gene-sequence determination. The identification of ESTs has proceeded rapidly, with approximately 74.2 million ESTs now available in public databases. EST approaches have largely been superseded by whole genome and transcriptome sequencing and metagenome sequencing.
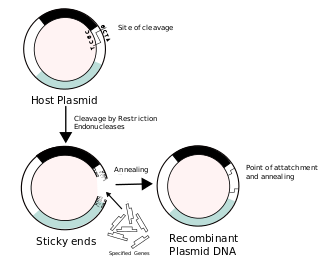
Recombinant DNA (rDNA) molecules are DNA molecules formed by laboratory methods of genetic recombination that bring together genetic material from multiple sources, creating sequences that would not otherwise be found in the genome.

Robert G. Roeder is an American biochemist. He is known as a pioneer scientist in eukaryotic transcription. He discovered three distinct nuclear RNA polymerases in 1969 and characterized many proteins involved in the regulation of transcription, including basic transcription factors and the first mammalian gene-specific activator over five decades of research. He is the recipient of the Gairdner Foundation International Award in 2000, the Albert Lasker Award for Basic Medical Research in 2003, and the Kyoto Prize in 2021. He currently serves as Arnold and Mabel Beckman Professor and Head of the Laboratory of Biochemical and Molecular Biology at The Rockefeller University.
Patrick Cramer is a German chemist, structural biologist, and molecular systems biologist. In 2020, he was honoured to be an international member of the National Academy of Sciences. He became president of the Max Planck Society in June 2023.

Roger David Kornberg is an American biochemist and professor of structural biology at Stanford University School of Medicine. Kornberg was awarded the Nobel Prize in Chemistry in 2006 for his studies of the process by which genetic information from DNA is copied to RNA, "the molecular basis of eukaryotic transcription."

Carolyn Widney Greider is an American molecular biologist and Nobel laureate. She joined the University of California, Santa Cruz as a Distinguished Professor in the department of molecular, cell, and developmental biology in October 2020.

Vincent R. Racaniello is a Higgins Professor in the Department of Microbiology and Immunology at Columbia University's College of Physicians and Surgeons. He is a co-author of a textbook on virology, Principles of Virology.

Pre-B-cell leukemia transcription factor 1 is a protein that in humans is encoded by the PBX1 gene. The homologous protein in Drosophila is known as extradenticle, and causes changes in embryonic development.

Helen Margaret Blau is an American biologist and the Donald E. and Delia B. Baxter Foundation Professor and Director of the Baxter Laboratory for Stem Cell Biology at Stanford University School of Medicine. She is known for establishing the reversibility of the mammalian differentiated state. Her landmark papers showed that nuclear reprogramming and the activation of novel programs of gene expression were possible, overturning the prevailing view that the differentiated state was fixed and irreversible. Her discoveries opened the door for cellular reprogramming and its application to stem cell biology.

Lydia Villa-Komaroff is a molecular and cellular biologist who has been an academic laboratory scientist, a university administrator, and a business woman. She was the third Mexican-American woman in the United States to receive a doctorate degree in the sciences (1975) and is a co-founding member of The Society for the Advancement of Chicanos/Hispanics and Native Americans in Science (SACNAS). Her most notable discovery was in 1978 during her post-doctoral research, when she was part of a team that discovered how bacterial cells could be used to generate insulin.

Don W. Cleveland is an American cancer biologist and neurobiologist.
Ruth Lehmann is a developmental and cell biologist. She is the Director of the Whitehead Institute for Biomedical Research. She previously was affiliated with the New York University School of Medicine, where she was the Director of the Skirball Institute of Biomolecular Medicine, the Laura and Isaac Perlmutter Professor of Cell Biology, and the Chair of the Department of Cell Biology. Her research focuses on germ cells and embryogenesis.
Ross C. Hardison is an American biochemist and molecular biologist, currently the T. Ming Chu Professor of Biochemistry and Molecular Biology at the Eberly College of Science, of the Pennsylvania State University.

Sir Richard Henry Treisman is a British scientist specialising in the molecular biology of cancer. Treisman is a director of research at the Francis Crick Institute in London.
Anindya Dutta is an Indian-born American biochemist and cancer researcher, a Chair of the Department of Genetics at the University of Alabama at Birmingham School of Medicine since 2021, who has served as Chair of the Department of Biochemistry and Molecular Genetics at the University of Virginia School of Medicine in 2011–2021. Dutta's research has focused on the mammalian cell cycle with an emphasis on DNA replication and repair and on noncoding RNAs. He is particularly interested in how de-regulation of these processes promote cancer progression. For his accomplishments he has been elected a Fellow of the American Association for the Advancement of Science, received the Ranbaxy Award in Biomedical Sciences, the Outstanding Investigator Award from the American Society for Investigative Pathology, the Distinguished Scientist Award from the University of Virginia and the Mark Brothers Award from the Indiana University School of Medicine.
Edward Francis Fritsch is a scientist in the field of molecular biology and cancer immunology.

Francisco Ernesto (Tito) Baralle is an Argentinian geneticist best known for his innovations in molecular biology and in particular the discovery of how genes are processed and mechanisms in mRNA splicing.
Robin Elizabeth Reed was an American professor of cell biology at the Harvard Medical School. Her research considered the molecular mechanisms that underpin neurodegenerative disease.










