
Sirolimus, also known as rapamycin and sold under the brand name Rapamune among others, is a macrolide compound that is used to coat coronary stents, prevent organ transplant rejection, treat a rare lung disease called lymphangioleiomyomatosis, and treat perivascular epithelioid cell tumor (PEComa). It has immunosuppressant functions in humans and is especially useful in preventing the rejection of kidney transplants. It is a mechanistic target of rapamycin (mTOR) kinase inhibitor that reduces the sensitivity of T cells and B cells to interleukin-2 (IL-2), inhibiting their activity.

Everolimus, sold under the brand name Afinitor among others, is a medication used as an immunosuppressant to prevent rejection of organ transplants and as a targeted therapy in the treatment of renal cell cancer and other tumours.

The mammalian target of rapamycin (mTOR), also referred to as the mechanistic target of rapamycin, and sometimes called FK506-binding protein 12-rapamycin-associated protein 1 (FRAP1), is a kinase that in humans is encoded by the MTOR gene. mTOR is a member of the phosphatidylinositol 3-kinase-related kinase family of protein kinases.
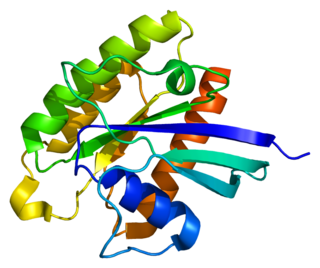
RHEB also known as Ras homolog enriched in brain (RHEB) is a GTP-binding protein that is ubiquitously expressed in humans and other mammals. The protein is largely involved in the mTOR pathway and the regulation of the cell cycle.

Ribosomal protein S6 kinase beta-1 (S6K1), also known as p70S6 kinase, is an enzyme that in humans is encoded by the RPS6KB1 gene. It is a serine/threonine kinase that acts downstream of PIP3 and phosphoinositide-dependent kinase-1 in the PI3 kinase pathway. As the name suggests, its target substrate is the S6 ribosomal protein. Phosphorylation of S6 induces protein synthesis at the ribosome.

Regulatory-associated protein of mTOR also known as raptor or KIAA1303 is an adapter protein that is encoded in humans by the RPTOR gene. Two mRNAs from the gene have been identified that encode proteins of 1335 and 1177 amino acids long.

Rapamycin-insensitive companion of mammalian target of rapamycin (RICTOR) is a protein that in humans is encoded by the RICTOR gene.

ULK1 is an enzyme that in humans is encoded by the ULK1 gene.
AuTophaGy related 1 (Atg1) is a 101.7kDa serine/threonine kinase in S.cerevisiae, encoded by the gene ATG1. It is essential for the initial building of the autophagosome and Cvt vesicles. In a non-kinase role it is - through complex formation with Atg13 and Atg17 - directly controlled by the TOR kinase, a sensor for nutrient availability.

Target of rapamycin complex subunit LST8, also known as mammalian lethal with SEC13 protein 8 (mLST8) or TORC subunit LST8 or G protein beta subunit-like, is a protein that in humans is encoded by the MLST8 gene. It is a subunit of both mTORC1 and mTORC2, complexes that regulate cell growth and survival in response to nutrient, energy, redox, and hormonal signals. It is upregulated in several human colon and prostate cancer cell lines and tissues. Knockdown of mLST8 prevented mTORC formation and inhibited tumor growth and invasiveness.

mTOR inhibitors are a class of drugs that inhibit the mammalian target of rapamycin (mTOR), which is a serine/threonine-specific protein kinase that belongs to the family of phosphatidylinositol-3 kinase (PI3K) related kinases (PIKKs). mTOR regulates cellular metabolism, growth, and proliferation by forming and signaling through two protein complexes, mTORC1 and mTORC2. The most established mTOR inhibitors are so-called rapalogs, which have shown tumor responses in clinical trials against various tumor types.

mTORC1, also known as mammalian target of rapamycin complex 1 or mechanistic target of rapamycin complex 1, is a protein complex that functions as a nutrient/energy/redox sensor and controls protein synthesis.
mTOR Complex 2 (mTORC2) is an acutely rapamycin-insensitive protein complex formed by serine/threonine kinase mTOR that regulates cell proliferation and survival, cell migration and cytoskeletal remodeling. The complex itself is rather large, consisting of seven protein subunits. The catalytic mTOR subunit, DEP domain containing mTOR-interacting protein (DEPTOR), mammalian lethal with sec-13 protein 8, and TTI1/TEL2 complex are shared by both mTORC2 and mTORC1. Rapamycin-insensitive companion of mTOR (RICTOR), mammalian stress-activated protein kinase interacting protein 1 (mSIN1), and protein observed with rictor 1 and 2 (Protor1/2) can only be found in mTORC2. Rictor has been shown to be the scaffold protein for substrate binding to mTORC2.

David M. Sabatini is an American scientist and a former professor of biology at the Massachusetts Institute of Technology. From 2002 to 2021, he was a member of the Whitehead Institute for Biomedical Research. He was also an investigator of the Howard Hughes Medical Institute from 2008 to 2021 and was elected to the National Academy of Sciences in 2016. He is known for his contributions in the areas of cell signaling and cancer metabolism, most notably the co-discovery of mTOR.

Michael Nip Hall is an American-Swiss molecular biologist and professor at the Biozentrum of the University of Basel, Switzerland. He discovered TOR, a protein central for regulating cell growth.

DNA-damage-inducible transcript 4 like (DDIT4L) or regulated in development and DNA damage response 2 (REDD2) is a protein that in humans is encoded by the DDIT4L gene. The gene is located on chromosome 4 or chromosome 3 in human or mouse respectively.
Immunometabolism is a branch of biology that studies the interplay between metabolism and immunology in all organisms. In particular, immunometabolism is the study of the molecular and biochemical underpinninngs for i) the metabolic regulation of immune function, and ii) the regulation of metabolism by molecules and cells of the immune system. Further categorization includes i) systemic immunometabolism and ii) cellular immunometabolism. Immunometabolism includes metabolic inflammation:a chronic, systemic, low grade inflammation, orchestrated by metabolic deregulation caused by obesity or aging.
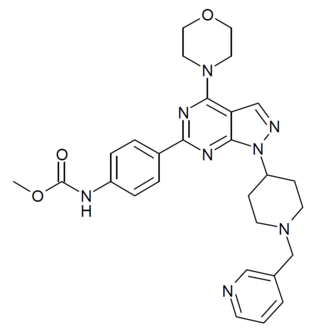
WYE-687 is a drug which acts as an inhibitor of both subtypes of the mechanistic target of rapamycin (mTOR), mTORC1 and mTORC2. It is being researched for potential applications in the treatment of various forms of cancer.

XL-388 is a drug which acts as a potent and selective inhibitor of both subtypes of the mechanistic target of rapamycin (mTOR), mTORC1 and mTORC2. It is being researched for the treatment of various forms of cancer, and has also been used to demonstrate a potential application for mTOR inhibitors in the treatment of neuropathic pain.
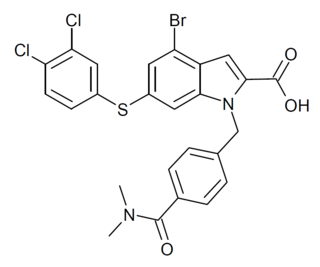
HY-124798 (Rheb inhibitor NR1) is the first compound to be developed that acts as a potent and selective inhibitor of Rheb, a GTP-binding protein which acts as an endogenous activator of the mechanistic target of rapamycin (mTOR) subtype mTORC1. Since many of the side effects of rapamycin and its analogues are thought to result from binding to the other subtype mTORC2, it is hoped that selective inhibition of mTORC1 should have a more selective effects profile. As mTORC1 and mTORC2 have binding sites that are very similar in structure, it has been challenging to develop highly subtype selective inhibitors, making indirect inhibition via modulation of other messenger proteins such as Rheb an attractive approach. However, since HY-124798 has a relatively weak IC50 of 2.1μM, and Rheb also has other targets in addition to mTORC1, it remains to be established whether it will deliver the hoped for improvements in pharmacological profile.

















