
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands".
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula O−2. The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature. Molecular oxygen (dioxygen) is a diradical containing two unpaired electrons, and superoxide results from the addition of an electron which fills one of the two degenerate molecular orbitals, leaving a charged ionic species with a single unpaired electron and a net negative charge of −1. Both dioxygen and the superoxide anion are free radicals that exhibit paramagnetism. Superoxide was historically also known as "hyperoxide".
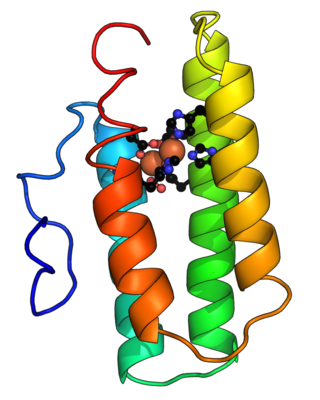
Hemerythrin (also spelled haemerythrin; Ancient Greek: αἷμα, romanized: haîma, lit. 'blood', Ancient Greek: ἐρυθρός, romanized: erythrós, lit. 'red') is an oligomeric protein responsible for oxygen (O2) transport in the marine invertebrate phyla of sipunculids, priapulids, brachiopods, and in a single annelid worm genus, Magelona. Myohemerythrin is a monomeric O2-binding protein found in the muscles of marine invertebrates. Hemerythrin and myohemerythrin are essentially colorless when deoxygenated, but turn a violet-pink in the oxygenated state.

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins.

Vaska's complex is the trivial name for the chemical compound trans-carbonylchlorobis(triphenylphosphine)iridium(I), which has the formula IrCl(CO)[P(C6H5)3]2. This square planar diamagnetic organometallic complex consists of a central iridium atom bound to two mutually trans triphenylphosphine ligands, carbon monoxide and a chloride ion. The complex was first reported by J. W. DiLuzio and Lauri Vaska in 1961. Vaska's complex can undergo oxidative addition and is notable for its ability to bind to O2 reversibly. It is a bright yellow crystalline solid.

Salen refers to a tetradentate C2-symmetric ligand synthesized from salicylaldehyde (sal) and ethylenediamine (en). It may also refer to a class of compounds, which are structurally related to the classical salen ligand, primarily bis-Schiff bases. Salen ligands are notable for coordinating a wide range of different metals, which they can often stabilise in various oxidation states. For this reason salen-type compounds are used as metal deactivators. Metal salen complexes also find use as catalysts.

In coordination chemistry, hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated. In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity, and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands.

Transition metal dinitrogen complexes are coordination compounds that contain transition metals as ion centers the dinitrogen molecules (N2) as ligands.

In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.

Salcomine is a coordination complex derived from the salen ligand and cobalt. The complex, which is planar, and a variety of its derivatives are carriers for O2 as well as oxidation catalysts.

Potassium peroxochromate, potassium tetraperoxochromate(V), or simply potassium perchromate, is an inorganic compound having the chemical formula K3[Cr(O2)4]. It is a red-brown paramagnetic solid. It is the potassium salt of tetraperoxochromate(V), one of the few examples of chromium in the +5 oxidation state and one of the rare examples of a complex stabilized only by peroxide ligands. This compound is used as a source of singlet oxygen.
A transition metal oxo complex is a coordination complex containing an oxo ligand. Formally O2-, an oxo ligand can be bound to one or more metal centers, i.e. it can exist as a terminal or (most commonly) as bridging ligands (Fig. 1). Oxo ligands stabilize high oxidation states of a metal. They are also found in several metalloproteins, for example in molybdenum cofactors and in many iron-containing enzymes. One of the earliest synthetic compounds to incorporate an oxo ligand is potassium ferrate (K2FeO4), which was likely prepared by Georg E. Stahl in 1702.
In organometallic chemistry, metal sulfur dioxide complexes are complexes that contain sulfur dioxide, SO2, bonded to a transition metal. Such compounds are common but are mainly of theoretical interest. Historically, the study of these compounds has provided insights into the mechanisms of migratory insertion reactions.

A metal salen complex is a coordination compound between a metal cation and a ligand derived from N,N′-bis(salicylidene)ethylenediamine, commonly called salen. The classical example is salcomine, the complex with divalent cobalt Co2+, usually denoted as Co(salen). These complexes are widely investigated as catalysts and enzyme mimics.

A transition metal fullerene complex is a coordination complex wherein fullerene serves as a ligand. Fullerenes are typically spheroidal carbon compounds, the most prevalent being buckminsterfullerene, C60.
In organometallic chemistry, a transition metal alkene complex is a coordination compound containing one or more alkene ligands. The inventory is large. Such compounds are intermediates in many catalytic reactions that convert alkenes to other organic products.
The Mukaiyama hydration is an organic reaction involving formal addition of an equivalent of water across an olefin by the action of catalytic bis(acetylacetonato)cobalt(II) complex, phenylsilane and atmospheric oxygen to produce an alcohol with Markovnikov selectivity.

Metal peroxides are metal-containing compounds with ionically- or covalently-bonded peroxide (O2−
2) groups. This large family of compounds can be divided into ionic and covalent peroxide. The first class mostly contains the peroxides of the alkali and alkaline earth metals whereas the covalent peroxides are represented by such compounds as hydrogen peroxide and peroxymonosulfuric acid (H2SO5). In contrast to the purely ionic character of alkali metal peroxides, peroxides of transition metals have a more covalent character.

Water oxidation catalysis (WOC) is the acceleration (catalysis) of the conversion of water into oxygen and protons:

Transition metal carboxylate complexes are coordination complexes with carboxylate (RCO2−) ligands. Reflecting the diversity of carboxylic acids, the inventory of metal carboxylates is large. Many are useful commercially, and many have attracted intense scholarly scrutiny. Carboxylates exhibit a variety of coordination modes, most common are κ1- (O-monodentate), κ2 (O,O-bidentate), and bridging.




![Structure of [Co(salen)(dmf)]2O2. DOESCF10.svg](http://upload.wikimedia.org/wikipedia/commons/thumb/8/8d/DOESCF10.svg/220px-DOESCF10.svg.png)















