
A sclerotium, is a compact mass of hardened fungal mycelium containing food reserves. One role of sclerotia is to survive environmental extremes. In some higher fungi such as ergot, sclerotia become detached and remain dormant until favorable growth conditions return. Sclerotia initially were mistaken for individual organisms and described as separate species until Louis René Tulasne proved in 1853 that sclerotia are only a stage in the life cycle of some fungi. Further investigation showed that this stage appears in many fungi belonging to many diverse groups. Sclerotia are important in the understanding of the life cycle and reproduction of fungi, as a food source, as medicine, and in agricultural blight management.
Tolypocladium inflatum is an ascomycete fungus originally isolated from a Norwegian soil sample that, under certain conditions, produces the immunosuppressant drug ciclosporin. In its sexual stage (teleomorph) it is a parasite on scarab beetles. It forms a small, compound ascocarp that arises from the cadaver of its host beetle. In its asexual stage (anamorph) it is a white mold that grows on soil. It is much more commonly found in its asexual stage and this is the stage that was originally given the name Tolypocladium inflatum.

Trichoderma is a genus of fungi in the family Hypocreaceae that is present in all soils, where they are the most prevalent culturable fungi. Many species in this genus can be characterized as opportunistic avirulent plant symbionts. This refers to the ability of several Trichoderma species to form mutualistic endophytic relationships with several plant species. The genomes of several Trichoderma specieshave been sequenced and are publicly available from the JGI.

Epichloë is a genus of ascomycete fungi forming an endophytic symbiosis with grasses. Grass choke disease is a symptom in grasses induced by some Epichloë species, which form spore-bearing mats (stromata) on tillers and suppress the development of their host plant's inflorescence. For most of their life cycle however, Epichloë grow in the intercellular space of stems, leaves, inflorescences, and seeds of the grass plant without incurring symptoms of disease. In fact, they provide several benefits to their host, including the production of different herbivore-deterring alkaloids, increased stress resistance, and growth promotion.

Acrophialophora fusispora is a poorly studied ascomycete fungus found in soil, air and various plants. A. fusispora is morphologically similar to the genera Paecilomyces and Masonia, but differ in the presence of pigmented conidiophores, verticillate phialides, and frequent sympodial proliferation. Moreover, A. fusispora is distinguished by its pigmented spindle-shaped conidia, covered with spiral bands. The fungus is naturally found in soils of tropical to temperate regions. The fungus has been identified as a plant and animal pathogen, and has recently been recognized as an emerging opportunistic human pathogen. A. fusispora infection in human is rare and has few documented clinical cases, but due to the rarity of the fungus and potential misidentification, the infections may be underdiagnosed. Clinical cases of A. fusispora include cases of keratitis, pulmonary colonization and infection, and cerebral infections. The fungus also has two documented cases of infection in dogs.

Geotrichum candidum is a fungus which is a member of the human microbiome, notably associated with skin, sputum, and faeces where it occurs in 25–30% of specimens. It is common in soil and has been isolated from soil collected around the world, in all continents.
Aspergillus ochraceus is a mold species in the genus Aspergillus known to produce the toxin ochratoxin A, one of the most abundant food-contaminating mycotoxins, and citrinin. It also produces the dihydroisocoumarin mellein. It is a filamentous fungus in nature and has characteristic biseriate conidiophores. Traditionally a soil fungus, has now began to adapt to varied ecological niches, like agricultural commodities, farmed animal and marine species. In humans and animals the consumption of this fungus produces chronic neurotoxic, immunosuppressive, genotoxic, carcinogenic and teratogenic effects. Its airborne spores are one of the potential causes of asthma in children and lung diseases in humans. The pig and chicken populations in the farms are the most affected by this fungus and its mycotoxins. Certain fungicides like mancozeb, copper oxychloride, and sulfur have inhibitory effects on the growth of this fungus and its mycotoxin producing capacities.
Coniochaeta hoffmannii, also known as Lecythophora hoffmannii, is an ascomycete fungus that grows commonly in soil. It has also been categorized as a soft-rot fungus capable of bringing the surface layer of timber into a state of decay, even when safeguarded with preservatives. Additionally, it has pathogenic properties, although it causes serious infection only in rare cases. A plant pathogen lacking a known sexual state, C. hoffmannii has been classified as a "dematiaceous fungus" despite its contradictory lack of pigmentation; both in vivo and in vitro, there is no correlation between its appearance and its classification.
Thielavia subthermophila is a ubiquitous, filamentous fungus that is a member of the phylum Ascomycota and order Sordariales. Known to be found on plants of arid environments, it is an endophyte with thermophilic properties, and possesses dense, pigmented mycelium. Thielavia subthermophila has rarely been identified as a human pathogen, with a small number of clinical cases including ocular and brain infections. For treatment, antifungal drugs such as amphotericin B have been used topically or intravenously, depending upon the condition.
Trichoderma longibrachiatum is a fungus in the genus Trichoderma. In addition to being a distinct species, T. longibrachiatum also typifies one of several clades within Trichoderma which comprises 21 different species. Trichoderma longibrachiatum is a soil fungus which is found all over the world but mainly in warmer climates. Many species from this clade have been adopted in various industries because of their ability to secrete large amounts of protein and metabolites.

Penicillium digitatum is a mesophilic fungus found in the soil of citrus-producing areas. It is a major source of post-harvest decay in fruits and is responsible for the widespread post-harvest disease in Citrus fruit known as green rot or green mould. In nature, this necrotrophic wound pathogen grows in filaments and reproduces asexually through the production of conidiophores and conidia. However, P. digitatum can also be cultivated in the laboratory setting. Alongside its pathogenic life cycle, P. digitatum is also involved in other human, animal and plant interactions and is currently being used in the production of immunologically based mycological detection assays for the food industry.
Aspergillus unguis is a species of fungus in the genus Aspergillus, and the asexual state (anamorph) of Emericella unguis. Aspergillus unguis is a filamentous soil-borne fungus found on decomposing plant matter and other moist substrates including with building materials and household dust. Aspergillus unguis occurs mainly in tropical and subtropical soils but has also been isolated from various marine and aquatic habitats. The species was first isolated in 1935 by Weill and L. Gaudin. Historically, A. unguis was assigned to the A. nidulans group, a common group of soil-borne fungi due to the resemblance of its ascospores and cleistothecia to those of Emericella nidulans. Aspergillus unguis is distinctive, however, in possessing spicular hyphae. A number of synonyms have been collapsed into this species, including Sterigmatocystis unguis, Aspergillus laokiashanensis and Aspergillus mellinus.

Aspergillus clavatus is a species of fungus in the genus Aspergillus with conidia dimensions 3–4.5 x 2.5–4.5 μm. It is found in soil and animal manure. The fungus was first described scientifically in 1834 by the French mycologist John Baptiste Henri Joseph Desmazières.
Paecilomyces marquandii is a soil-borne filamentous fungus distributed throughout temperate to tropical latitudes worldwide including forest, grassland, sewage sludge and strongly metal polluted area characterized by high tolerance in heavy metals. Simultaneous toxic action of zinc and alachlor result an increase in uptake of metal in this fungus but disrupts the cell membrane. Paecilomyces marquandii is known to parasitize the mushroom, Cuphophyllus virgineus, in the family, Hygrophoraceae. Paecilomyces marquandii is categorised as a biosafety risk group 1 in Canada and is not thought to be a significant pathogen of humans or animals.

Mariannaea elegans an anamorphic fungus. It is mainly found on rotting wood and soil. M. elegans is not pathogenic to humans, animals, or plants.
Botryotrichum piluliferum is a fungal species first identified in 1885 by Saccardo and Marchal. It was discovered to be the asexual state of a member of the ascomycete genus, Chaetomium. The name B. piluliferum now applies to the fungus in all its states. B. piluliferum has been found worldwide in a wide range of habitats such as animal dung and vegetation. The colonies of this fungus start off white and grow rapidly to a brown colour. The conidia are smooth and white. B. piluliferum grows optimally at a temperature of 25-30 °C and a pH of 5.5.
Microascus manginii is a filamentous fungal species in the genus Microascus. It produces both sexual (teleomorph) and asexual (anamorph) reproductive stages known as M. manginii and Scopulariopsis candida, respectively. Several synonyms appear in the literature because of taxonomic revisions and re-isolation of the species by different researchers. M. manginii is saprotrophic and commonly inhabits soil, indoor environments and decaying plant material. It is distinguishable from closely related species by its light colored and heart-shaped ascospores used for sexual reproduction. Scopulariopsis candida has been identified as the cause of some invasive infections, often in immunocompromised hosts, but is not considered a common human pathogen. There is concern about amphotericin B resistance in S. candida.
Aspergillus giganteus is a species of fungus in the genus Aspergillus that grows as a mold. It was first described in 1901 by Wehmer, and is one of six Aspergillus species from the Clavati section of the subgenus Fumigati. Its closest taxonomic relatives are Aspergillus rhizopodus and Aspergillus longivescia.
A mycoparasite is an organism with the ability to parasitize fungi.
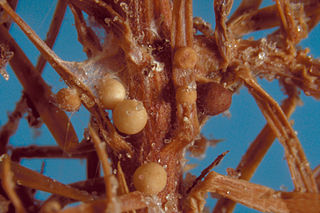
Agroathelia rolfsii is a corticioid fungus in the order Amylocorticiales. It is a facultative plant pathogen and is the causal agent of "southern blight" disease in crops.










