Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propane.

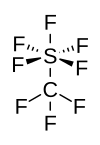
Sulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF6. It is a colorless, odorless, non-flammable, and non-toxic gas. SF
6 has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. It is a hypervalent molecule.

Hydrofluorocarbons (HFCs) are man-made organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air conditioning and as refrigerants; R-134a (1,1,1,2-tetrafluoroethane) is one of the most commonly used HFC refrigerants. In order to aid the recovery of the stratospheric ozone layer, HFCs were adopted to replace the more potent chlorofluorocarbons (CFCs), which were phased out from use by the Montreal Protocol, and hydrochlorofluorocarbons (HCFCs) which are presently being phased out. HFCs replaced older chlorofluorocarbons such as R-12 and hydrochlorofluorocarbons such as R-21. HFCs are also used in insulating foams, aerosol propellants, as solvents and for fire protection.
Difluoromethane, also called difluoromethylene, HFC-32Methylene Fluoride or R-32, is an organic compound of the dihalogenoalkane variety. It has the formula of CH2F2. It is a colorless gas in the ambient atmosphere and is slightly soluble in the water, with a high thermal stability. Due to the low melting and boiling point, (-136.0 °C and -51.6 °C respectively) contact with this compound may result in frostbite. In the United States, the Clean Air Act Section 111 on Volatile Organic Compounds (VOC) has listed difluoromethane as an exception (since 1997) from the definition of VOC due to its low production of tropospheric ozone. Difluoromethane is commonly used in endothermic processes such as refrigeration or air conditioning.

The infrared atmospheric window refers to a region of the infrared spectrum where there is relatively little absorption of terrestrial thermal radiation by atmospheric gases. The window plays an important role in the atmospheric greenhouse effect by maintaining the balance between incoming solar radiation and outgoing IR to space. In the Earth's atmosphere this window is roughly the region between 8 and 14 μm although it can be narrowed or closed at times and places of high humidity because of the strong absorption in the water vapor continuum or because of blocking by clouds. It covers a substantial part of the spectrum from surface thermal emission which starts at roughly 5 μm. Principally it is a large gap in the absorption spectrum of water vapor. Carbon dioxide plays an important role in setting the boundary at the long wavelength end. Ozone partly blocks transmission in the middle of the window.

Tetrafluoromethane, also known as carbon tetrafluoride or R-14, is the simplest perfluorocarbon (CF4). As its IUPAC name indicates, tetrafluoromethane is the perfluorinated counterpart to the hydrocarbon methane. It can also be classified as a haloalkane or halomethane. Tetrafluoromethane is a useful refrigerant but also a potent greenhouse gas. It has a very high bond strength due to the nature of the carbon–fluorine bond.

Nitrogen trifluoride is an inorganic, colorless, non-flammable, toxic gas with a slightly musty odor. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. Nitrogen trifluoride is also an extremely strong and long-lived greenhouse gas. Its atmospheric burden exceeded 2 parts per trillion during 2019 and has doubled every five years since the late 20th century.
Fluoroform, or trifluoromethane, is the chemical compound with the formula CHF3. It is a hydrofluorocarbon as well as being apart of the haloforms, a class of compounds with the formula CHX3 with C3v symmetry. Fluoroform is used in diverse applications in organic synthesis. It is not an ozone depleter but is a greenhouse gas.

Disulfur decafluoride is a chemical compound with the formula S2F10. It was discovered in 1934 by Denbigh and Whytlaw-Gray. Each sulfur atom of the S2F10 molecule is octahedral, and surrounded by five fluorine atoms and one sulfur atom. The two sulfur atoms are connected by a single bond. In the S2F10 molecule, the oxidation state of each sulfur atoms is +5, but their valency is 6. S2F10 is highly toxic, with toxicity four times that of phosgene.

Sulfur tetrafluoride is the chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.
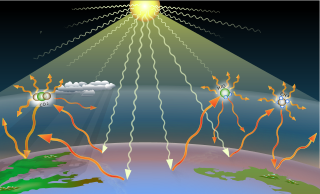
Greenhouse gases are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. What distinguishes them from other gases is that they absorb the wavelengths of radiation that a planet emits, resulting in the greenhouse effect. The Earth is warmed by sunlight, causing its surface to radiate heat, which is then mostly absorbed by water vapor (H2O), carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and ozone (O3). Without greenhouse gases, the average temperature of Earth's surface would be about −18 °C (0 °F), rather than the present average of 15 °C (59 °F).

Thionyl tetrafluoride, also known as sulfur tetrafluoride oxide, is an inorganic compound with the formula SOF4. It is a colorless gas.

Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.
Fluorinated gases (F-gases) are chemical compounds containing fluorine that are gases near room temperature.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
Difluoroamino sulfur pentafluoride is a gaseous chemical compound of fluorine, sulfur, and nitrogen. It is unusual in having a hexa-coordinated sulfur atom with a link to nitrogen. Other names for this substance include difluoro(pentafluorosulfur)amine, pentafluorosulfanyldifluoramine, and pentafluorosulfanyl N,N-difluoramine.

C4-FN (C4-fluoronitrile, C4FN) is a perfluorinated compound developed as a high-dielectric gas for high-voltage switchgear. It has the structure (CF3)2CFC≡N, which can be described as perfluoroisobutyronitrile, falling under the category of PFAS, or per- and polyfluoroalkyl substances.