
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine. On several scales other than the revised Pauling scale, nitrogen's electronegativity is also listed as greater than chlorine's, such as on the Allen, Allred-Rochow, Martynov-Batsanov, Mulliken-Jaffe, Nagle, and Noorizadeh-Shakerzadeh electronegativity scales.

Sulfuric acid (American spelling) or sulphuric acid (Commonwealth spelling), also known as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula H2SO4. It is a colorless, odorless and viscous liquid that is miscible with water.

Aqua regia is a mixture of nitric acid and hydrochloric acid, optimally in a molar ratio of 1:3. Aqua regia is a yellow-orange fuming liquid, so named by alchemists because it can dissolve the noble metals gold and platinum, though not all metals.
Chloroform, or trichloromethane, is an organic compound with formula CHCl3. It is a colorless, strong-smelling, dense liquid that is produced on a large scale as a precursor to PTFE. It is also a precursor to various refrigerants. It is one of the four chloromethanes and a trihalomethane. It is a powerful anesthetic, euphoriant, anxiolytic and sedative when inhaled or ingested.

Ethylene oxide is an organic compound with the formula C
2H
4O. It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of silver catalyst.

Iron(III) chloride is the inorganic compound with the formula. Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The color depends on the viewing angle: by reflected light the crystals appear dark green, but by transmitted light they appear purple-red.

Dinitrogen pentoxide is the chemical compound with the formula N2O5, also known as nitrogen pentoxide or nitric anhydride. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that melt at 41 °C. Its boiling point is 47 °C, and sublimes slightly above room temperature, yielding a colorless gas.

Triphenylmethane, or triphenyl methane, is the hydrocarbon with the formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic skeleton of many synthetic dyes called triarylmethane dyes, many of them are pH indicators, and some display fluorescence. A trityl group in organic chemistry is a triphenylmethyl group Ph3C, e.g. triphenylmethyl chloride (trityl chloride) and the triphenylmethyl radical (trityl radical).
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine. The chloroalkane class provides common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious.

A single-displacement reaction, also known as single replacement reaction or exchange reaction, is a chemical reaction in which one element is replaced by another in a compound.
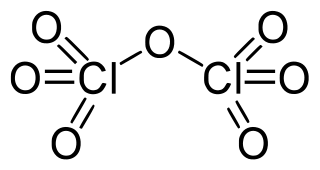
Dichlorine heptoxide is the chemical compound with the formula Cl2O7. This chlorine oxide is the anhydride of perchloric acid. It is produced by the careful distillation of perchloric acid in the presence of the dehydrating agent phosphorus pentoxide:

Gold(III) chloride, traditionally called auric chloride, is a compound of gold and chlorine with the molecular formula Au2Cl6. The "III" in the name indicates the gold has an oxidation state of +3, which is typical for many gold compounds. Gold(III) chloride is hygroscopic and decomposes in visible light. This compound is a dimer of AuCl3. This compound has few uses, although it does catalyze a variety of organic reactions.
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapidly inserts into other bonds.
Tetrafluoroethylene (TFE) is a fluorocarbon with the chemical formula C2F4. It is the simplest perfluorinated alkene. This gaseous species is used primarily in the industrial preparation of fluoropolymers.

Sodium formate, HCOONa, is the sodium salt of formic acid, HCOOH. It usually appears as a white deliquescent powder.
Dehydrohalogenation is an elimination reaction that eliminates (removes) a hydrogen halide from a substrate. The reaction is usually associated with the synthesis of alkenes, but it has wider applications.
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete. An intermediate is the reaction product of each of these steps, except for the last one, which forms the final product. Reactive intermediates are usually short lived and are very seldom isolated. Also, owing to the short lifetime, they do not remain in the product mixture.

Dichlorofluoromethane or Freon 21 or R 21 is a halomethane or hydrochlorofluorocarbon with the formula CHCl2F. It is a colorless and odorless gas. It is produced by fluorination of chloroform using a catalyst such as antimony trifluoride:

Fluorine perchlorate, also called perchloryl hypofluorite is the rarely encountered chemical compound of fluorine, chlorine, and oxygen with the chemical formula ClO
4F or FOClO
3. It is an extremely unstable gas that explodes spontaneously and has a penetrating odor.

Trinitroethylorthocarbonate also known as TNEOC is an oxidizer with excellent chemical stability. Its explosion point is 238 °C, and it begins to be decomposed at 200 °C. Its explosion heat is 5.797 J/g and specific volume is 694 L/kg. Its structure is closely related to that of trinitroethylorthoformate (TNEOF). Both are highly explosive and very shock-sensitive, and may be dissolved in nitroalkanes to reduce their shock-sensitivity.















