
Ubiquitin is a small regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ubiquitously. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Four genes in the human genome code for ubiquitin: UBB, UBC, UBA52 and RPS27A.

A degron is a portion of a protein that is important in regulation of protein degradation rates. Known degrons include short amino acid sequences, structural motifs and exposed amino acids located anywhere in the protein. In fact, some proteins can even contain multiple degrons. Degrons are present in a variety of organisms, from the N-degrons first characterized in yeast to the PEST sequence of mouse ornithine decarboxylase. Degrons have been identified in prokaryotes as well as eukaryotes. While there are many types of different degrons, and a high degree of variability even within these groups, degrons are all similar for their involvement in regulating the rate of a protein's degradation. Much like protein degradation mechanisms are categorized by their dependence or lack thereof on Ubiquitin, a small protein involved in proteasomal protein degradation, Degrons may also be referred to as “Ubiquitin-dependent" or “Ubiquitin-independent".

Presenilin-1(PS-1) is a presenilin protein that in humans is encoded by the PSEN1 gene. Presenilin-1 is one of the four core proteins in the gamma secretase complex, which is considered to play an important role in generation of amyloid beta (Aβ) from amyloid-beta precursor protein (APP). Accumulation of amyloid beta is associated with the onset of Alzheimer's disease.

Proteasome subunit alpha type-3 also known as macropain subunit C8 and proteasome component C8 is a protein that in humans is encoded by the PSMA3 gene. This protein is one of the 17 essential subunits that contributes to the complete assembly of 20S proteasome complex.

Proteasome subunit alpha type-4 also known as macropain subunit C9, proteasome component C9, and 20S proteasome subunit alpha-3 is a protein that in humans is encoded by the PSMA4 gene. This protein is one of the 17 essential subunits that contributes to the complete assembly of 20S proteasome complex.

Proteasome subunit beta type-1 also known as 20S proteasome subunit beta-6 is a protein that in humans is encoded by the PSMB1 gene. This protein is one of the 17 essential subunits that contributes to the complete assembly of 20S proteasome complex. In particular, proteasome subunit beta type-1, along with other beta subunits, assemble into two heptameric rings and subsequently a proteolytic chamber for substrate degradation. The eukaryotic proteasome recognized degradable proteins, including damaged proteins for protein quality control purpose or key regulatory protein components for dynamic biological processes. An essential function of a modified proteasome, the immunoproteasome, is the processing of class I MHC peptides.

Proteasome subunit beta type-10 as known as 20S proteasome subunit beta-2i is a protein that in humans is encoded by the PSMB10 gene.

Proteasome subunit beta type-3, also known as 20S proteasome subunit beta-3, is a protein that in humans is encoded by the PSMB3 gene. This protein is one of the 17 essential subunits that contribute to the complete assembly of the 20S proteasome complex. In particular, proteasome subunit beta type-2, along with other beta subunits, assemble into two heptameric rings and subsequently a proteolytic chamber for substrate degradation. The eukaryotic proteasome recognizes degradable proteins, including damaged proteins for protein quality control purpose or key regulatory protein components for dynamic biological processes.

Presenilin-2 is a protein that is encoded by the PSEN2 gene.

Proteasome subunit alpha type-6 is a protein that in humans is encoded by the PSMA6 gene. This protein is one of the 17 essential subunits that contributes to the complete assembly of 20S proteasome complex.

Proteasome subunit alpha type-7 also known as 20S proteasome subunit alpha-4 is a protein that in humans is encoded by the PSMA7 gene. This protein is one of the 17 essential subunits that contributes to the complete assembly of 20S proteasome complex.
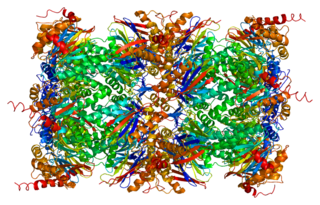
Proteasome subunit beta type-5 as known as 20S proteasome subunit beta-5 is a protein that in humans is encoded by the PSMB5 gene. This protein is one of the 17 essential subunits that contributes to the complete assembly of 20S proteasome complex. In particular, proteasome subunit beta type-5, along with other beta subunits, assemble into two heptameric rings and subsequently a proteolytic chamber for substrate degradation. This protein contains "chymotrypsin-like" activity and is capable of cleaving after large hydrophobic residues of peptide. The eukaryotic proteasome recognized degradable proteins, including damaged proteins for protein quality control purpose or key regulatory protein components for dynamic biological processes. An essential function of a modified proteasome, the immunoproteasome, is the processing of class I MHC peptides.
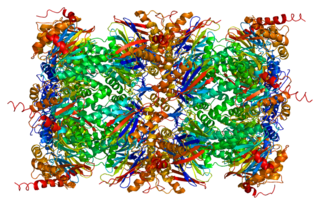
Proteasome subunit alpha type-5 also known as 20S proteasome subunit alpha-5 is a protein that in humans is encoded by the PSMA5 gene. This protein is one of the 17 essential subunits that contributes to the complete assembly of 20S proteasome complex.

26S proteasome non-ATPase regulatory subunit 1, also as known as 26S Proteasome Regulatory Subunit Rpn2, is a protein that in humans is encoded by the PSMD1 gene. This protein is one of the 19 essential subunits that contributes to the complete assembly of 19S proteasome complex.

26S proteasome non-ATPase regulatory subunit 2, also as known as 26S Proteasome Regulatory Subunit Rpn1, is an enzyme that in humans is encoded by the PSMD2 gene.

E3 ubiquitin-protein ligase SMURF1 is an enzyme that in humans is encoded by the SMURF1 gene. The SMURF1 Gene encodes a protein with a size of 757 amino acids and the molecular mass of this protein is 86114 Da.

Homocysteine-responsive endoplasmic reticulum-resident ubiquitin-like domain member 1 protein is a protein that in humans is encoded by the HERPUD1 gene.

Ubiquilin-2 is a protein that in humans is encoded by the UBQLN2 gene.

26S proteasome non-ATPase regulatory subunit 14, also known as 26S proteasome non-ATPase subunit Rpn11, is an enzyme that in humans is encoded by the PSMD14 gene. This protein is one of the 19 essential subunits of the complete assembled 19S proteasome complex. Nine subunits Rpn3, Rpn5, Rpn6, Rpn7, Rpn8, Rpn9, Rpn11, SEM1, and Rpn12 form the lid sub complex of the 19S regulatory particle of the proteasome complex.
























