Related Research Articles

In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells.
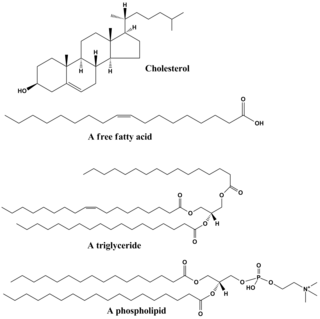
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology.

Lipolysis is the metabolic pathway through which lipid triglycerides are hydrolyzed into a glycerol and free fatty acids. It is used to mobilize stored energy during fasting or exercise, and usually occurs in fat adipocytes. The most important regulatory hormone in lipolysis is insulin; lipolysis can only occur when insulin action falls to low levels, as occurs during fasting. Other hormones that affect lipolysis include leptin, glucagon, epinephrine, norepinephrine, growth hormone, atrial natriuretic peptide, brain natriuretic peptide, and cortisol.

4-Hydroxynonenal, or 4-hydroxy-2E-nonenal or 4-hydroxy-2-nonenal or 4-HNE or HNE,, is an α,β-unsaturated hydroxyalkenal that is produced by lipid peroxidation in cells. 4-HNE is the primary α,β-unsaturated hydroxyalkenal formed in this process. It is a colorless oil. It is found throughout animal tissues, and in higher quantities during oxidative stress due to the increase in the lipid peroxidation chain reaction, due to the increase in stress events. 4-HNE has been hypothesized to play a key role in cell signal transduction, in a variety of pathways from cell cycle events to cellular adhesion.
Mycolic acids are long fatty acids found in the cell walls of Mycobacteriales taxon, a group of bacteria that includes Mycobacterium tuberculosis, the causative agent of the disease tuberculosis. They form the major component of the cell wall of many Mycobacteriales species. Despite their name, mycolic acids have no biological link to fungi; the name arises from the filamentous appearance their presence gives Mycobacteriales under high magnification. The presence of mycolic acids in the cell wall also gives Mycobacteriales a distinct gross morphological trait known as "cording". Mycolic acids were first isolated by Stodola et al. in 1938 from an extract of M. tuberculosis.
Triacsin C is an inhibitor of long fatty acyl CoA synthetase that has been isolated from Streptomyces aureofaciens. It blocks β-cell apoptosis, induced by fatty acids (lipoapoptosis) in a rat model of obesity. In addition, it blocks the de novo synthesis of triglycerides, diglycerides, and cholesterol esters, thus interfering with lipid metabolism.

Sterol regulatory element-binding proteins (SREBPs) are transcription factors that bind to the sterol regulatory element DNA sequence TCACNCCAC. Mammalian SREBPs are encoded by the genes SREBF1 and SREBF2. SREBPs belong to the basic-helix-loop-helix leucine zipper class of transcription factors. Unactivated SREBPs are attached to the nuclear envelope and endoplasmic reticulum membranes. In cells with low levels of sterols, SREBPs are cleaved to a water-soluble N-terminal domain that is translocated to the nucleus. These activated SREBPs then bind to specific sterol regulatory element DNA sequences, thus upregulating the synthesis of enzymes involved in sterol biosynthesis. Sterols in turn inhibit the cleavage of SREBPs and therefore synthesis of additional sterols is reduced through a negative feed back loop.

Sterol regulatory element-binding protein cleavage-activating protein, also known as SREBP cleavage-activating protein or SCAP, is a protein that in humans is encoded by the SCAP gene.

Stearoyl-CoA desaturase (Δ-9-desaturase) is an endoplasmic reticulum enzyme that catalyzes the rate-limiting step in the formation of monounsaturated fatty acids (MUFAs), specifically oleate and palmitoleate from stearoyl-CoA and palmitoyl-CoA. Oleate and palmitoleate are major components of membrane phospholipids, cholesterol esters and alkyl-diacylglycerol. In humans, the enzyme is encoded by the SCD gene.

Valosin-containing protein (VCP) or transitional endoplasmic reticulum ATPase also known as p97 in mammals and CDC48 in S. cerevisiae, is an enzyme that in humans is encoded by the VCP gene. The TER ATPase is an ATPase enzyme present in all eukaryotes and archaebacteria. Its main function is to segregate protein molecules from large cellular structures such as protein assemblies, organelle membranes and chromatin, and thus facilitate the degradation of released polypeptides by the multi-subunit protease proteasome.

Autocrine motility factor receptor, isoform 2 is a protein that in humans is encoded by the AMFR gene.

Adipose differentiation-related protein, also known as perilipin 2, ADRP or adipophilin, is a protein which belongs to the perilipin (PAT) family of cytoplasmic lipid droplet (CLD)–binding proteins. In humans it is encoded by the ADFP gene. This protein surrounds the lipid droplet along with phospholipids and is involved in assisting the storage of neutral lipids within the lipid droplets.

Adipose triglyceride lipase, also known as patatin-like phospholipase domain-containing protein 2 and ATGL, is an enzyme that in humans is encoded by the PNPLA2 gene. ATGL catalyses the first reaction of lipolysis, where triacylglycerols are hydrolysed to diacylglycerols.
Lipid droplets, also referred to as lipid bodies, oil bodies or adiposomes, are lipid-rich cellular organelles that regulate the storage and hydrolysis of neutral lipids and are found largely in the adipose tissue. They also serve as a reservoir for cholesterol and acyl-glycerols for membrane formation and maintenance. Lipid droplets are found in all eukaryotic organisms and store a large portion of lipids in mammalian adipocytes. Initially, these lipid droplets were considered to merely serve as fat depots, but since the discovery in the 1990s of proteins in the lipid droplet coat that regulate lipid droplet dynamics and lipid metabolism, lipid droplets are seen as highly dynamic organelles that play a very important role in the regulation of intracellular lipid storage and lipid metabolism. The role of lipid droplets outside of lipid and cholesterol storage has recently begun to be elucidated and includes a close association to inflammatory responses through the synthesis and metabolism of eicosanoids and to metabolic disorders such as obesity, cancer, and atherosclerosis. In non-adipocytes, lipid droplets are known to play a role in protection from lipotoxicity by storage of fatty acids in the form of neutral triacylglycerol, which consists of three fatty acids bound to glycerol. Alternatively, fatty acids can be converted to lipid intermediates like diacylglycerol (DAG), ceramides and fatty acyl-CoAs. These lipid intermediates can impair insulin signaling, which is referred to as lipid-induced insulin resistance and lipotoxicity. Lipid droplets also serve as platforms for protein binding and degradation. Finally, lipid droplets are known to be exploited by pathogens such as the hepatitis C virus, the dengue virus and Chlamydia trachomatis among others.

A diglyceride, or diacylglycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Two possible forms exist, 1,2-diacylglycerols and 1,3-diacylglycerols. Diglycerides are natural components of food fats, though minor in comparison to triglycerides. DAGs can act as surfactants and are commonly used as emulsifiers in processed foods. DAG-enriched oil has been investigated extensively as a fat substitute due to its ability to suppress the accumulation of body fat; with total annual sales of approximately USD 200 million in Japan since its introduction in the late 1990s till 2009.
In molecular biology, the UBX protein domain is found in ubiquitin-regulatory proteins, which are members of the ubiquitination pathway, as well as a number of other ubiquitin-like proteins including FAF-1, the human Rep-8 reproduction protein and several hypothetical proteins from yeast. The function of the UBX domain is not known although the fragment of avian FAF-1 containing the UBX domain causes apoptosis of transfected cells.

Lipase inhibitors belong to a drug class that is used as an antiobesity agent. Their mode of action is to inhibit gastric and pancreatic lipases, enzymes that play an important role in the digestion of dietary fat. Lipase inhibitors are classified in the ATC-classification system as A08AB . Numerous compounds have been either isolated from nature, semi-synthesized, or fully synthesized and then screened for their lipase inhibitory activity but the only lipase inhibitor on the market is orlistat . Lipase inhibitors have also shown anticancer activity, by inhibiting fatty acid synthase.

Fas associated factor family member 2 is a protein that in humans is encoded by the FAF2 gene.

Perilipin 5, also known as Oxpatperilipin 5 or PLIN5, is a protein that belongs to perilipin family. This protein group has been shown to be responsible for lipid droplet's biogenesis, structure and degradation. In particular, Perilipin 5 is a lipid droplet-associated protein whose function is to keep the balance between lipolysis and lipogenesis, as well as maintaining lipid droplet homeostasis. For example, in oxidative tissues, muscular tissues and cardiac tissues, PLIN5 promotes association between lipid droplets and mitochondria.
Hypoxia inducible lipid droplet-associated is a protein that in humans is encoded by the HILPDA gene.
References
- ↑ Imai, Yukiho; Nakada, Akiko; Hashida, Ryoichi; Sugita, Yuji; Tanaka, Toshio; Tsujimoto, Gozoh; Matsumoto, Kenji; Akasawa, Akira; Saito, Hirohisa; Oshida, Tadahilo (October 2002). "Cloning and characterization of the highly expressed ETEA gene from blood cells of atopic dermatitis patients". Biochemical and Biophysical Research Communications. 297 (5): 1282–1290. doi:10.1016/S0006-291X(02)02380-X. PMID 12372427.
- ↑ Hofmann, Kay; Bucher, Philipp (May 1996). "The UBA domain: a sequence motif present in multiple enzyme classes of the ubiquitination pathway". Trends in Biochemical Sciences. 21 (5): 172–173. doi:10.1016/s0968-0004(96)30015-7. ISSN 0968-0004. PMID 8871400.
- 1 2 Buchberger, A (2002-05-01). "From UBA to UBX: new words in the ubiquitin vocabulary". Trends in Cell Biology. 12 (5): 216–221. doi:10.1016/S0962-8924(02)02269-9. PMID 12062168.
- ↑ Kim, Hyeonwoo; Zhang, Hong; Meng, David; Russell, Geoffrey; Lee, Joon No; Ye, Jin (August 2013). "UAS domain of Ubxd8 and FAF1 polymerizes upon interaction with long-chain unsaturated fatty acids". Journal of Lipid Research. 54 (8): 2144–2152. doi: 10.1194/jlr.M037218 . PMC 3708364 . PMID 23720822.
- ↑ Halawani, Dalia; Latterich, Martin (June 2006). "p97: The Cell's Molecular Purgatory?". Molecular Cell. 22 (6): 713–717. doi: 10.1016/j.molcel.2006.06.003 . PMID 16793541.
- ↑ Olzmann, James A.; Richter, Caleb M.; Kopito, Ron R. (2013-01-22). "Spatial regulation of UBXD8 and p97/VCP controls ATGL-mediated lipid droplet turnover". Proceedings of the National Academy of Sciences. 110 (4): 1345–1350. Bibcode:2013PNAS..110.1345O. doi: 10.1073/pnas.1213738110 . ISSN 0027-8424. PMC 3557085 . PMID 23297223.
- ↑ Lee, Joon No; Kim, Hyeonwoo; Yao, Hongbing; Chen, Yan; Weng, Kayson; Ye, Jin (2010-12-14). "Identification of Ubxd8 protein as a sensor for unsaturated fatty acids and regulator of triglyceride synthesis". Proceedings of the National Academy of Sciences. 107 (50): 21424–21429. Bibcode:2010PNAS..10721424L. doi: 10.1073/pnas.1011859107 . ISSN 0027-8424. PMC 3003070 . PMID 21115839.
- ↑ Zheng, Jing; Cao, Yu; Yang, Jun; Jiang, Hui (2022-10-06). "UBXD8 mediates mitochondria-associated degradation to restrain apoptosis and mitophagy". EMBO Reports. 23 (10): e54859. doi:10.15252/embr.202254859. ISSN 1469-221X. PMC 9535754 . PMID 35979733.
- ↑ Ye, Jin (October 2012). "Cellular responses to unsaturated fatty acids mediated by their sensor Ubxd8". Frontiers in Biology. 7 (5): 397–403. doi:10.1007/s11515-012-1247-6. ISSN 1674-7984. S2CID 15917436.
- ↑ Loregger, Anke; Raaben, Matthijs; Tan, Josephine; Scheij, Saskia; Moeton, Martina; van den Berg, Marlene; Gelberg-Etel, Hila; Stickel, Elmer; Roitelman, Joseph; Brummelkamp, Thijn; Zelcer, Noam (November 2017). "Haploid Mammalian Genetic Screen Identifies UBXD8 as a Key Determinant of HMGCR Degradation and Cholesterol Biosynthesis". Arteriosclerosis, Thrombosis, and Vascular Biology. 37 (11): 2064–2074. doi:10.1161/ATVBAHA.117.310002. ISSN 1079-5642. PMC 5671778 . PMID 28882874.
- ↑ Kim, Hyeonwoo; Zhang, Hong; Meng, David; Russell, Geoffrey; Lee, Joon No; Ye, Jin (August 2013). "UAS domain of Ubxd8 and FAF1 polymerizes upon interaction with long-chain unsaturated fatty acids". Journal of Lipid Research. 54 (8): 2144–2152. doi: 10.1194/jlr.M037218 . PMC 3708364 . PMID 23720822.
- ↑ Lee, Joon No; Kim, Hyeonwoo; Yao, Hongbing; Chen, Yan; Weng, Kayson; Ye, Jin (2010-12-14). "Identification of Ubxd8 protein as a sensor for unsaturated fatty acids and regulator of triglyceride synthesis". Proceedings of the National Academy of Sciences. 107 (50): 21424–21429. Bibcode:2010PNAS..10721424L. doi: 10.1073/pnas.1011859107 . ISSN 0027-8424. PMC 3003070 . PMID 21115839.
- ↑ Lee, Joon No; Zhang, Xiangyu; Feramisco, Jamison D.; Gong, Yi; Ye, Jin (November 2008). "Unsaturated Fatty Acids Inhibit Proteasomal Degradation of Insig-1 at a Postubiquitination Step". Journal of Biological Chemistry. 283 (48): 33772–33783. doi: 10.1074/jbc.M806108200 . PMC 2586246 . PMID 18835813.
- ↑ Ishiyama, Junichi; Taguchi, Ryoko; Akasaka, Yunike; Shibata, Saiko; Ito, Minoru; Nagasawa, Michiaki; Murakami, Koji (February 2011). "Unsaturated FAs prevent palmitate-induced LOX-1 induction via inhibition of ER stress in macrophages". Journal of Lipid Research. 52 (2): 299–307. doi: 10.1194/jlr.M007104 . PMC 3023550 . PMID 21078775.
- ↑ Zhao, Linlin; Wang, Shuqing; Zhu, Qianli; Wu, Bin; Liu, Zhijun; OuYang, Bo; Chou, James J. (September 2017). "Specific Interaction of the Human Mitochondrial Uncoupling Protein 1 with Free Long-Chain Fatty Acid". Structure. 25 (9): 1371–1379.e3. doi: 10.1016/j.str.2017.07.005 . PMID 28781081.