
Sertraline, sold under the brand name Zoloft among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. The efficacy of sertraline for depression is similar to that of other antidepressants, and the differences are mostly confined to side effects. Sertraline is better tolerated than the older tricyclic antidepressants. Sertraline is effective for panic disorder, social anxiety disorder, generalized anxiety disorder (GAD), and obsessive–compulsive disorder (OCD). However, for OCD, cognitive behavioral therapy, particularly in combination with sertraline, is a better treatment. Although approved for post-traumatic stress disorder (PTSD), sertraline leads to only modest improvement in this condition. Sertraline also alleviates the symptoms of premenstrual dysphoric disorder (PMDD) and can be used in sub-therapeutic doses or intermittently for its treatment.
Anorgasmia is a type of sexual dysfunction in which a person cannot achieve orgasm despite adequate stimulation. Anorgasmia is far more common in females than in males and is especially rare in younger men. The problem is greater in women who are post-menopausal. In males, it is most closely associated with delayed ejaculation. Anorgasmia can often cause sexual frustration.

Vaginal lubrication is a naturally produced fluid that lubricates the vagina. Vaginal lubrication is always present, but production increases significantly near ovulation and during sexual arousal in anticipation of sexual intercourse. Vaginal dryness is the condition in which this lubrication is insufficient, and sometimes artificial lubricants are used to augment it. Without sufficient lubrication, sexual intercourse can be painful. The vaginal lining has no glands, and therefore the vagina must rely on other methods of lubrication. Plasma from vaginal walls due to vascular engorgement is considered to be the chief lubrication source, and the Bartholin's glands, located slightly below and to the left and right of the introitus, also secrete mucus to augment vaginal-wall secretions. Near ovulation, cervical mucus provides additional lubrication.
Sexual arousal disorder is characterized by a lack or absence of sexual fantasies and desire for sexual activity in a situation that would normally produce sexual arousal, or the inability to attain or maintain typical responses to sexual arousal. The disorder is found in the DSM-IV. The condition should not be confused with a sexual desire disorder.
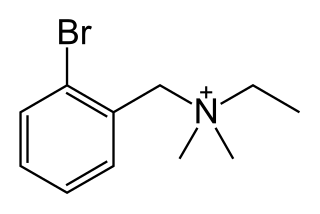
Bretylium (also bretylium tosylate) is an antiarrhythmic agent. It blocks the release of noradrenaline from nerve terminals. In effect, it decreases output from the peripheral sympathetic nervous system. It also acts by blocking K+ channels and is considered a class III antiarrhythmic. The dose is 5–10 mg/kg and side effects are high blood pressure followed by low blood pressure and ventricular ectopy.

Desmetramadol (INN), also known as O-desmethyltramadol (O-DSMT), is an opioid analgesic and the main active metabolite of tramadol. Tramadol is demethylated by the liver enzyme CYP2D6 to desmetramadol in the same way as codeine, and so similarly to the variation in effects seen with codeine, individuals who have a less active form of CYP2D6 will tend to have reduced analgesic effects from tramadol. Because desmetramadol itself does not need to be metabolized to induce an analgesic effect, it can be used in individuals with low CYP2D6 activity unlike tramadol.

Alpha-adrenergic agonists are a class of sympathomimetic agents that selectively stimulates alpha adrenergic receptors. The alpha-adrenergic receptor has two subclasses α1 and α2. Alpha 2 receptors are associated with sympatholytic properties. Alpha-adrenergic agonists have the opposite function of alpha blockers. Alpha adrenoreceptor ligands mimic the action of epinephrine and norepinephrine signaling in the heart, smooth muscle and central nervous system, with norepinephrine being the highest affinity. The activation of α1 stimulates the membrane bound enzyme phospholipase C, and activation of α2 inhibits the enzyme adenylate cyclase. Inactivation of adenylate cyclase in turn leads to the inactivation of the secondary messenger cyclic adenosine monophosphate and induces smooth muscle and blood vessel constriction.

8-OH-DPAT is a research chemical of the aminotetralin chemical class which was developed in the 1980s and has been widely used to study the function of the 5-HT1A receptor. It was one of the first major 5-HT1A receptor full agonists to be discovered.

RB-101 is a drug that acts as an enkephalinase inhibitor, which is used in scientific research.

7-Nitroindazole, or 7-NI, is a heterocyclic small molecule containing an indazole ring that has been nitrated at the 7 position. Nitroindazole acts as a selective inhibitor for neuronal nitric oxide synthase, a hemoprotein enzyme that, in neuronal tissue, converts arginine to citrulline and nitric oxide (NO). Nitric oxide can diffuse through the plasma membrane into neighbouring cells, allowing cell signalling, so nitroindazole indirectly inhibits this signalling process. Other inhibitors exist such as 3-bromo-7-nitroindazole, which is more potent but less specific, or NPA (N-propyl-L-arginine), which acts on a different site.

Flesinoxan (DU-29,373) is a potent and selective 5-HT1A receptor partial/near-full agonist of the phenylpiperazine class. Originally developed as a potential antihypertensive drug, flesinoxan was later found to possess antidepressant and anxiolytic effects in animal tests. As a result, it was investigated in several small human pilot studies for the treatment of major depressive disorder, and was found to have robust effectiveness and very good tolerability. However, due to "management decisions", the development of flesinoxan was stopped and it was not pursued any further.

CI-988 (PD-134,308) is a drug which acts as a cholecystokinin antagonist, selective for the CCKB subtype. In animal studies it showed anxiolytic effects and potentiated the analgesic action of both morphine and endogenous opioid peptides, as well as preventing the development of tolerance to opioids and reducing symptoms of withdrawal. Consequently, it was hoped that it might have clinical applications for the treatment of pain and anxiety in humans, but trial results were disappointing with only minimal therapeutic effects observed even at high doses. The reason for the failure of CI-988 and other CCKB antagonists in humans despite their apparent promise in pre-clinical animal studies is unclear, although poor pharmacokinetic properties of the currently available drugs are a possible explanation, and CCKB antagonists are still being researched for possible uses as adjuvants to boost the activity of other drugs.
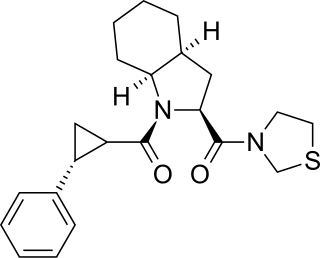
S-17092 is a drug which acts as a selective inhibitor of the enzyme prolyl endopeptidase. This enzyme is involved in the metabolic breakdown of a number of neuropeptide neurotransmitters in the brain, and so inhibiting the action of the enzyme increases the activity of these neuropeptides. This produces nootropic effects which make S-17092 a promising and novel treatment for neurodegenerative conditions such as Alzheimer's disease and Parkinson's disease.

Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs that are typically used as antidepressants in the treatment of major depressive disorder, anxiety disorders, and other psychological conditions.
Sexual anhedonia, also known as pleasure dissociative orgasmic disorder, is a condition in which an individual cannot feel pleasure from an orgasm. It is thought to be a variant of hypoactive sexual desire disorder.
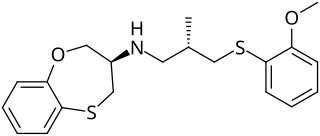
F15845 is a cardiac drug proposed to have beneficial effects for the treatment of angina pectoris, arrhythmias and ischemia by inhibiting the persistent sodium current. The drug, currently in phase II of clinical trials, targets the persistent sodium current with selectivity and produces minimal adverse effects in current experimental studies.
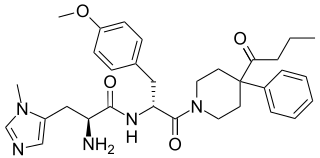
BMS-470539 is a small-molecule experimental drug which acts as a potent and highly selective full agonist of the MC1 receptor. It was discovered in 2003 as part of an effort to understand the role of the MC1 receptor in immunomodulation, and has since been used in scientific research to determine its role in inflammatory processes. The compound was designed with the intention of mimicking the central His-Phe-Arg-Trp pharmacophore of the melanocortins, and this proved to be successful based on its favorable pharmacodynamic profile.

o-Phenyl-3-iodotyramine (o-PIT) is a drug which acts as a selective agonist for the trace amine-associated receptor 1. It has reasonable selectivity for TAAR1 but relatively low potency, and is rapidly metabolised in vivo, making it less useful for research than newer ligands such as RO5166017.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.
BW284C51 is a selective acetylcholinesterase inhibitor. It is also a nicotinic antagonist.















