Boranes is the name given to compounds with the formula BxHy and related anions. Many such boranes are known. Most common are those with 1 to 12 boron atoms. Although they have few practical applications, the boranes exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes are also well developed.
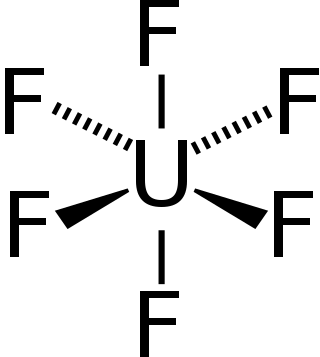
Uranium hexafluoride (UF6), (sometimes called "hex") is an inorganic compound with the formula UF6. Uranium hexafluoride is a volatile white solid that reacts with water, releasing corrosive hydrofluoric acid. The compound reacts mildly with aluminium, forming a thin surface layer of AlF3 that resists any further reaction from the compound. UF6 is used in the process of enriching uranium, which produces fuel for nuclear reactors and nuclear weapons.

Gaseous diffusion is a technology that was used to produce enriched uranium by forcing gaseous uranium hexafluoride (UF6) through microporous membranes. This produces a slight separation (enrichment factor 1.0043) between the molecules containing uranium-235 (235U) and uranium-238 (238U). By use of a large cascade of many stages, high separations can be achieved. It was the first process to be developed that was capable of producing enriched uranium in industrially useful quantities, but is nowadays considered obsolete, having been superseded by the more-efficient gas centrifuge process.

The term scorpionate ligand refers to a tridentate (three-donor-site) ligand which would bind to a metal in a fac manner. The most popular class of scorpionates are the hydrotris(pyrazolyl)borates or Tp ligands. These were also the first to become popular. These ligands first appeared in journals in 1966 from the then little-known DuPont chemist of Ukrainian descent, Swiatoslaw Trofimenko. Trofimenko called this discovery "a new and fertile field of remarkable scope".

In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix octa. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, [Co(NH3)6]3+, which is not octahedral in the mathematical sense due to the orientation of the N−H bonds, is referred to as octahedral.
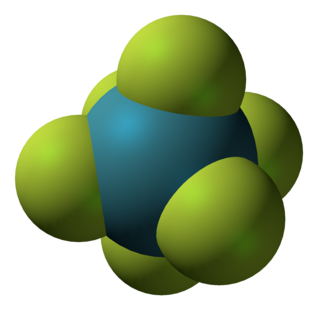
Xenon hexafluoride is a noble gas compound with the formula XeF6. It is one of the three binary fluorides of xenon that have been studied experimentally, the other two being XeF2 and XeF4. All known are exergonic and stable at normal temperatures. XeF6 is the strongest fluorinating agent of the series. It is a colorless solid that readily sublimes into intensely yellow vapors.

Uranyl nitrate is a water-soluble yellow uranium salt with the formula UO2(NO3)2 · n H2O. The hexa-, tri-, and dihydrates are known. The compound is mainly of interest because it is an intermediate in the preparation of nuclear fuels.

Uranium tetrafluoride is the inorganic compound with the formula UF4. It is a green solid with an insignificant vapor pressure and low solubility in water. Uranium in its tetravalent (uranous) state is important in various technological processes. In the uranium refining industry it is known as green salt.
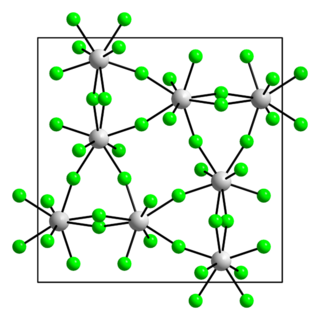
Uranium pentafluoride is the inorganic compound with the chemical formula UF5. It is a pale yellow paramagnetic solid. The compound has attracted interest because it is related to uranium hexafluoride, which is widely used to produce uranium fuel. It crystallizes in two polymorphs, called α- and β-UF5.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.

Borohydride refers to the anion [BH4]−, which is also called tetrahydroborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing [BH4−nXn]−, where n is an integer from 0 to 3, for example cyanoborohydride or cyanotrihydroborate [BH3(CN)]− and triethylborohydride or triethylhydroborate [BH(CH2CH3)3]−. Borohydrides find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known. Tetrahydroborates are also of academic and industrial interest in inorganic chemistry.
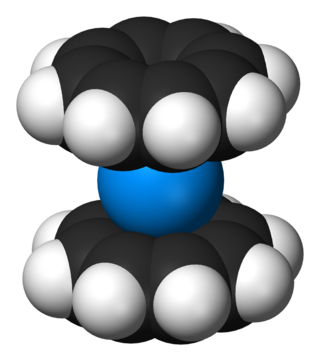
Organouranium chemistry is the science exploring the properties, structure and reactivity of organouranium compounds, which are organometallic compounds containing a carbon to uranium chemical bond. The field is of some importance to the nuclear industry and of theoretical interest in organometallic chemistry.

Digallane is an inorganic compound with the chemical formula GaH
2(H)
2GaH
2. It is the dimer of the monomeric compound gallane. The eventual preparation of the pure compound, reported in 1989, was hailed as a "tour de force." Digallane had been reported as early as 1941 by Wiberg; however, this claim could not be verified by later work by Greenwood and others. This compound is a colorless gas that decomposes above 0 °C.

Plutonium hexafluoride is the highest fluoride of plutonium, and is of interest for laser enrichment of plutonium, in particular for the production of pure plutonium-239 from irradiated uranium. This pure plutonium is needed to avoid premature ignition of low-mass nuclear weapon designs by neutrons produced by spontaneous fission of plutonium-240.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.
William Taylor Miller was an American professor of organic chemistry at Cornell University. His experimental research included investigations into the mechanism of addition of halogens, especially fluorine, to hydrocarbons. His work focused primarily on the physical and chemical properties of fluorocarbons and chlorofluorocarbons, and the synthesis of novel electrophilic reagents.
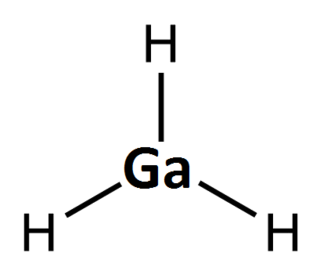
Gallane, also systematically named trihydridogallium, is an inorganic compound of gallium with the chemical formula GaH
3. It is a photosensitive, colourless gas that cannot be concentrated in pure form. Gallane is both the simplest member of the gallanes, and the prototype of the monogallanes. It has no economic uses, and is only intentionally produced for academic reasons.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
Jaqueline Kiplinger is an American inorganic chemist who specializes in organometallic actinide chemistry. Over the course of her career, she has done extensive work with fluorocarbons and actinides. She is currently a Fellow of the Materials Synthesis and Integrated Devices group in the Materials Physics and Applications Division of Los Alamos National Laboratory (LANL). Her current research interests are focused on the development of chemistry for the United States’ national defense and energy needs.
Diphosphorus tetrafluoride is a gaseous compound of phosphorus and fluorine with formula P2F4. Two fluorine atoms are connected to each phosphorus atom, and there is a bond between the two phosphorus atoms. Phosphorus can be considered to have oxidation state +2, as indicated by the name phosphorus difluoride.