Related Research Articles
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants are frequently added to industrial products, such as polymers, fuels, and lubricants, to extend their usable lifetimes. Foods are also treated with antioxidants to forestall spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress.
Senescence or biological aging is the gradual deterioration of functional characteristics in living organisms. The word senescence can refer to either cellular senescence or to senescence of the whole organism. Organismal senescence involves an increase in death rates and/or a decrease in fecundity with increasing age, at least in the later part of an organism's life cycle. However, the resulting effects of senescence can be delayed. The 1934 discovery that calorie restriction can extend lifespans by 50% in rats, the existence of species having negligible senescence, and the existence of potentially immortal organisms such as members of the genus Hydra have motivated research into delaying senescence and thus age-related diseases. Rare human mutations can cause accelerated aging diseases.
Life extension is the concept of extending the human lifespan, either modestly through improvements in medicine or dramatically by increasing the maximum lifespan beyond its generally-settled limit of 125 years. Several researchers in the area, along with "life extensionists", "immortalists", or "longevists", postulate that future breakthroughs in tissue rejuvenation, stem cells, regenerative medicine, molecular repair, gene therapy, pharmaceuticals, and organ replacement will eventually enable humans to have indefinite lifespans through complete rejuvenation to a healthy youthful condition (agerasia). The ethical ramifications, if life extension becomes a possibility, are debated by bioethicists.

Glutathione peroxidase (GPx) is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. The biochemical function of glutathione peroxidase is to reduce lipid hydroperoxides to their corresponding alcohols and to reduce free hydrogen peroxide to water.
The free radical theory of aging states that organisms age because cells accumulate free radical damage over time. A free radical is any atom or molecule that has a single unpaired electron in an outer shell. While a few free radicals such as melanin are not chemically reactive, most biologically relevant free radicals are highly reactive. For most biological structures, free radical damage is closely associated with oxidative damage. Antioxidants are reducing agents, and limit oxidative damage to biological structures by passivating them from free radicals.

In chemistry and biology, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (O2), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (O2H), superoxide (O2-), hydroxyl radical (OH.), and singlet oxygen. ROS are pervasive because they are readily produced from O2, which is abundant. ROS are important in many ways, both beneficial and otherwise. ROS function as signals, that turn on and off biological functions. They are intermediates in the redox behavior of O2, which is central to fuel cells. ROS are central to the photodegradation of organic pollutants in the atmosphere. Most often however, ROS are discussed in a biological context, ranging from their effects on aging and their role in causing dangerous genetic mutations.

Biogerontology is the sub-field of gerontology concerned with the biological aging process, its evolutionary origins, and potential means to intervene in the process. The term "biogerontology" was coined by S. Rattan, and came in regular use with the start of the journal BIOGERONTOLOGY in 2000. It involves interdisciplinary research on the causes, effects, and mechanisms of biological aging. Biogerontologist Leonard Hayflick has said that the natural average lifespan for a human is around 92 years and, if humans do not invent new approaches to treat aging, they will be stuck with this lifespan. James Vaupel has predicted that life expectancy in industrialized countries will reach 100 for children born after the year 2000. Many surveyed biogerontologists have predicted life expectancies of more than three centuries for people born after the year 2100. Other scientists, more controversially, suggest the possibility of unlimited lifespans for those currently living. For example, Aubrey de Grey offers the "tentative timeframe" that with adequate funding of research to develop interventions in aging such as strategies for engineered negligible senescence, "we have a 50/50 chance of developing technology within about 25 to 30 years from now that will, under reasonable assumptions about the rate of subsequent improvements in that technology, allow us to stop people from dying of aging at any age". The idea of this approach is to use presently available technology to extend lifespans of currently living humans long enough for future technological progress to resolve any remaining aging-related issues. This concept has been referred to as longevity escape velocity.

Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal redox state of cells can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. Oxidative stress from oxidative metabolism causes base damage, as well as strand breaks in DNA. Base damage is mostly indirect and caused by the reactive oxygen species generated, e.g., O2− (superoxide radical), OH (hydroxyl radical) and H2O2 (hydrogen peroxide). Further, some reactive oxidative species act as cellular messengers in redox signaling. Thus, oxidative stress can cause disruptions in normal mechanisms of cellular signaling.
Enquiry into the evolution of ageing, or aging, aims to explain why a detrimental process such as ageing would evolve, and why there is so much variability in the lifespans of organisms. The classical theories of evolution suggest that environmental factors, such as predation, accidents, disease, and/or starvation, ensure that most organisms living in natural settings will not live until old age, and so there will be very little pressure to conserve genetic changes that increase longevity. Natural selection will instead strongly favor genes which ensure early maturation and rapid reproduction, and the selection for genetic traits which promote molecular and cellular self-maintenance will decline with age for most organisms.
Following is a list of topics related to life extension:

Michael Ristow is a German medical researcher who has published influential articles on biochemical aspects of mitochondrial metabolism and particularly the possibly health-promoting role of reactive oxygen species in diseases like type 2 diabetes, obesity and cancer, as well as general aging due to a process called mitohormesis.

Negligible senescence is a term coined by biogerontologist Caleb Finch to denote organisms that do not exhibit evidence of biological aging (senescence), such as measurable reductions in their reproductive capability, measurable functional decline, or rising death rates with age. There are many species where scientists have seen no increase in mortality after maturity. This may mean that the lifespan of the organism is so long that researchers' subjects have not yet lived up to the time when a measure of the species' longevity can be made. Turtles, for example, were once thought to lack senescence, but more extensive observations have found evidence of decreasing fitness with age.
David Gems is a British geneticist who studies the biology and genetics of ageing (biogerontology). He is Professor of Biogerontology at the Research Department of Genetics, Evolution and Environment, University College London and he is a co-founder and Research Director of the UCL Institute of Healthy Ageing. His work concerns understanding the underlying causes of aging. His research laboratory tests theories of aging and develops new ones using a short-lived animal model C. elegans.

Methionine sulfoxide is the organic compound with the formula CH3S(O)CH2CH2CH(NH2)CO2H. It is an amino acid that occurs naturally although it is formed post-translationally.

Biomarkers of aging are biomarkers that could predict functional capacity at some later age better than chronological age. Stated another way, biomarkers of aging would give the true "biological age", which may be different from the chronological age.

Selenium is an essential micronutrient for animals, though it is toxic in large doses. In plants, it sometimes occurs in toxic amounts as forage, e.g. locoweed. Selenium is a component of the amino acids selenocysteine and selenomethionine. In humans, selenium is a trace element nutrient that functions as cofactor for glutathione peroxidases and certain forms of thioredoxin reductase. Selenium-containing proteins are produced from inorganic selenium via the intermediacy of selenophosphate (PSeO33−).

The mitochondrial theory of ageing has two varieties: free radical and non-free radical. The first is one of the variants of the free radical theory of ageing. It was formulated by J. Miquel and colleagues in 1980 and was developed in the works of Linnane and coworkers (1989). The second was proposed by A. N. Lobachev in 1978.
This timeline lists notable events in the history of research into senescence or biological aging, including the research and development of life extension methods, brain aging delay methods and rejuvenation.

Helmut Sies is a German physician, biochemist and university professor. He was the first to demonstrate the existence of hydrogen peroxide as a normal attribute of aerobic life in 1970, and he introduced the concept of Oxidative stress in 1985. He also worked on the biological strategies of antioxidant defense and the biochemistry of nutritional antioxidants.
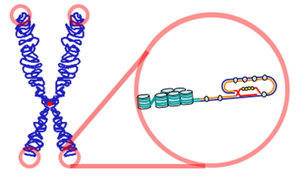
The relationship between telomeres and longevity and changing the length of telomeres is one of the new fields of research on increasing human lifespan and even human immortality. Telomeres are sequences at the ends of chromosomes that shorten with each cell division and determine the lifespan of cells. The telomere was first discovered by biologist Hermann Joseph Muller in the early 20th century. However, experiments by Elizabeth Blackburn, Carol Greider, and Jack Szostak in the 1980s led to the successful discovery of telomerase and a better understanding of telomeres.
References
- ↑ "Vadim Gladyshev | Brigham and Women's Hospital (BWH) | ResearchGate". ResearchGate. Retrieved 2017-11-29.
- ↑ Hatfield, Dolph L. (2016-07-01). "Redox Pioneer: Professor Vadim N. Gladyshev". Antioxidants & Redox Signaling. 25 (1): 1–9. doi:10.1089/ars.2015.6625. ISSN 1557-7716. PMC 4931753 . PMID 26984707.
- ↑ Poganik, J. R., Zhang, B., Baht, G. S., Tyshkovskiy, A., Deik, A., Kerepesi, C., ... & Gladyshev, V. N. (2022). Biological age is increased by stress and restored upon recovery. Cell Metabolism.
- ↑ Gladyshev, V. N. (2014). "The Free Radical Theory of Aging is Dead. Long Live the Damage Theory!". Antioxidants & Redox Signaling. 20 (4): 727–731. doi:10.1089/ars.2013.5228. PMC 3901353 . PMID 24159899.
- ↑ Hatfield, D. L. (2016). "Redox Pioneer: Professor Vadim N. Gladyshev". Antioxidants & Redox Signaling. 25 (1): 1–9. doi:10.1089/ars.2015.6625. PMC 4931753 . PMID 26984707.
- ↑ "You are what you eat: Old food shortens lifespan in animals". New Scientist. Retrieved 2017-11-29.
- ↑ Hatfield, Dolph L.; Gladyshev, Vadim N. (2002-06-01). "How Selenium Has Altered Our Understanding of the Genetic Code". Molecular and Cellular Biology. 22 (11): 3565–3576. doi:10.1128/MCB.22.11.3565-3576.2002. ISSN 0270-7306. PMC 133838 . PMID 11997494.
- ↑ "NIH Announces 2013 High-Risk, High-Reward Research Awards". National Institutes of Health (NIH). 2015-08-05. Retrieved 2017-11-29.
- ↑ "News from the National Academy of Sciences". 26 April 2021. Retrieved 2 July 2021.
Newly elected members and their affiliations at the time of election are: ... Gladyshev, Vadim N.; professor of medicine, Harvard Medical School; and director of Redox Medicine, Brigham and Women's Hospital, Boston
- ↑ Gladyshev, Vadim N. (2020-07-23). "The Ground Zero of Organismal Life and Aging". Trends in Molecular Medicine. 27 (1): 11–19. doi:10.1016/j.molmed.2020.08.012. PMC 9183202 . PMID 32980264.