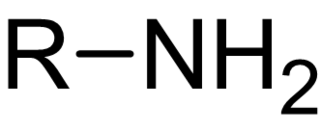
In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration.

Titration is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte. A reagent, termed the titrant or titrator, is prepared as a standard solution of known concentration and volume. The titrant reacts with a solution of analyte to determine the analyte's concentration. The volume of titrant that reacted with the analyte is termed the titration volume.

Nitrous acid is a weak and monoprotic acid known only in solution, in the gas phase, and in the form of nitrite salts. It was discovered by Carl Wilhelm Scheele, who called it "phlogisticated acid of niter". Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes.

William Howard Stein was an American biochemist who collaborated in the determination of the ribonuclease sequence, as well as how its structure relates to catalytic activity, earning a Nobel Prize in Chemistry in 1972 for his work. Stein was also involved in the invention of the automatic amino acid analyzer, an advancement in chromatography that opened the door to modern methods of chromatography, such as liquid chromatography and gas chromatography.

In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.

In chemistry, a chemical test is a qualitative or quantitative procedure designed to identify, quantify, or characterise a chemical compound or chemical group.
The Dakin–West reaction is a chemical reaction that transforms an amino-acid into a keto-amide using an acid anhydride and a base, typically pyridine. It is named for Henry Drysdale Dakin (1880–1952) and Randolph West (1890–1949). In 2016 Schreiner and coworkers reported the first asymmetric variant of this reaction employing short oligopeptides as catalysts.
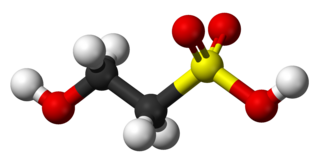
Isethionic acid is an organosulfur compound containing an alkylsulfonic acid located beta to a hydroxy group. Its discovery is generally attributed to Heinrich Gustav Magnus, who prepared it by the action of solid sulfur trioxide on ethanol in 1833. It is a white water-soluble solid used in the manufacture of certain surfactants and in the industrial production of taurine. It is most commonly available in the form of its sodium salt.

Phosphotungstic acid (PTA) or tungstophosphoric acid (TPA), is a heteropoly acid with the chemical formula H3PW12O40]. It forms hydrates H3[PW12O40]·nH2O. It is normally isolated as the n = 24 hydrate but can be desiccated to the hexahydrate (n = 6). EPTA is the name of ethanolic phosphotungstic acid, its alcohol solution used in biology. It has the appearance of small, colorless-grayish or slightly yellow-green crystals, with melting point 89 °C (24 H2O hydrate). It is odorless and soluble in water (200 g/100 ml). It is not especially toxic, but is a mild acidic irritant. The compound is known by a variety of names and acronyms (see 'other names' section of infobox).
The Lowry protein assay is a biochemical assay for determining the total level of protein in a solution. The total protein concentration is exhibited by a color change of the sample solution in proportion to protein concentration, which can then be measured using colorimetric techniques. It is named for the biochemist Oliver H. Lowry who developed the reagent in the 1940s. His 1951 paper describing the technique is the most-highly cited paper ever in the scientific literature, cited over 300,000 times.
The Kjeldahl method or Kjeldahl digestion (Danish pronunciation:[ˈkʰelˌtɛˀl]) in analytical chemistry is a method for the quantitative determination of a sample's organic nitrogen plus ammonia/ammonium. (NH3/NH4+). Without modification, other forms of inorganic nitrogen, for instance nitrate, are not included in this measurement. Using an empirical relation between Kjeldahl nitrogen and protein, it is an important method for indirectly quantifying protein content of a sample. This method was developed by Johan Kjeldahl in 1883.

Absolute configuration refers to the spatial arrangement of atoms within a chiral molecular entity and its resultant stereochemical description. Absolute configuration is typically relevant in organic molecules where carbon is bonded to four different substituents. This type of construction creates two possible enantiomers. Absolute configuration uses a set of rules to describe the relative positions of each bond around the chiral center atom. The most common labeling method uses the descriptors R or S and is based on the Cahn–Ingold–Prelog priority rules. R and S refer to rectus and sinister, Latin for right and left, respectively.
The Willard Gibbs Award, presented by the Chicago Section of the American Chemical Society, was established in 1910 by William A. Converse (1862–1940), a former Chairman and Secretary of the Chicago Section of the society and named for Professor Josiah Willard Gibbs (1839–1903) of Yale University. Gibbs, whose formulation of the Phase Rule founded a new science, is considered by many to be the only American-born scientist whose discoveries are as fundamental in nature as those of Newton and Galileo.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.

Donald Dexter Van Slyke, nicknamed Van, was a Dutch American biochemist. His achievements included the publication of 317 journal articles and 5 books, as well as numerous awards, among them the National Medal of Science and the first AMA Scientific Achievement Award. The Van Slyke determination, a test of amino acids, is named after him.
Charles Clifton Richardson is an American biochemist and professor at Harvard University. Richardson received his undergraduate education at Duke University, where he majored in medicine. He received his M.D. at Duke Medical School in 1960. Richardson works as a professor at Harvard Medical School, and he served as editor/associate editor of the Annual Review of Biochemistry from 1972 to 2003. Richardson received the American Chemical Society Award in Biological Chemistry in 1968, as well as numerous other accolades.

George Stark is an American chemist and biochemist. His research interests include protein and enzyme function and modification, interferons and cytokines, signal transduction, and gene expression.

Pauline Merritt Hald was an American clinical chemist and medical researcher, based in New Haven, Connecticut. She worked in the laboratory of chemist John P. Peters for many years, and published the first description of his flame photometry technique for measuring serum sodium and potassium levels.
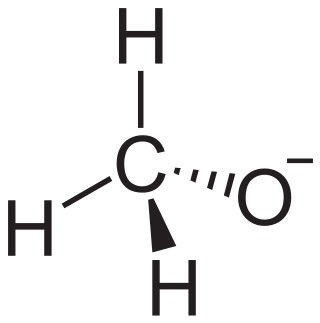
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as RO−, where R is the organyl substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis. Transition metal alkoxides are widely used for coatings and as catalysts.














