In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may be similar to that of gasoline or lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases, liquids, low melting solids or polymers.
Nitrogen fixation is a chemical process by which molecular nitrogen (N
2), which has a strong triple covalent bond, is converted into ammonia (NH
3) or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. The nitrogen in air is molecular dinitrogen, a relatively nonreactive molecule that is metabolically useless to all but a few microorganisms. Biological nitrogen fixation or diazotrophy is an important microbe-mediated process that converts dinitrogen (N2) gas to ammonia (NH3) using the nitrogenase protein complex (Nif).

The carbon cycle is that part of the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycle. Carbon is the main component of biological compounds as well as a major component of many minerals such as limestone. The carbon cycle comprises a sequence of events that are key to making Earth capable of sustaining life. It describes the movement of carbon as it is recycled and reused throughout the biosphere, as well as long-term processes of carbon sequestration (storage) to and release from carbon sinks.

The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmospheric, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems.

A biogeochemical cycle, or more generally a cycle of matter, is the movement and transformation of chemical elements and compounds between living organisms, the atmosphere, and the Earth's crust. Major biogeochemical cycles include the carbon cycle, the nitrogen cycle and the water cycle. In each cycle, the chemical element or molecule is transformed and cycled by living organisms and through various geological forms and reservoirs, including the atmosphere, the soil and the oceans. It can be thought of as the pathway by which a chemical substance cycles the biotic compartment and the abiotic compartments of Earth. The biotic compartment is the biosphere and the abiotic compartments are the atmosphere, lithosphere and hydrosphere.
The abiogenic petroleum origin hypothesis proposes that most of earth's petroleum and natural gas deposits were formed inorganically, commonly known as abiotic oil. Scientific evidence overwhelmingly supports a biogenic origin for most of the world's petroleum deposits. Mainstream theories about the formation of hydrocarbons on earth point to an origin from the decomposition of long-dead organisms, though the existence of hydrocarbons on extraterrestrial bodies like Saturn's moon Titan indicates that hydrocarbons are sometimes naturally produced by inorganic means. A historical overview of theories of the abiogenic origins of hydrocarbons has been published.
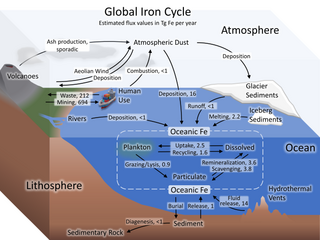
The iron cycle (Fe) is the biogeochemical cycle of iron through the atmosphere, hydrosphere, biosphere and lithosphere. While Fe is highly abundant in the Earth's crust, it is less common in oxygenated surface waters. Iron is a key micronutrient in primary productivity, and a limiting nutrient in the Southern ocean, eastern equatorial Pacific, and the subarctic Pacific referred to as High-Nutrient, Low-Chlorophyll (HNLC) regions of the ocean.

The hydrogen cycle consists of hydrogen exchanges between biotic (living) and abiotic (non-living) sources and sinks of hydrogen-containing compounds.
Marine chemistry, also known as ocean chemistry or chemical oceanography, is the study of chemical content in marine environments as influenced by plate tectonics and seafloor spreading, turbidity, currents, sediments, pH levels, atmospheric constituents, metamorphic activity, and ecology. Marine life has adapted to the chemistries unique to Earth's oceans, and marine ecosystems are sensitive to changes in ocean chemistry.

The mercury cycle is a biogeochemical cycle influenced by natural and anthropogenic processes that transform mercury through multiple chemical forms and environments.
The Guaymas Basin is the largest marginal rift basin located in the Gulf of California. It made up of the northern and southern trough and is linked to the Guaymas Fault to the north and the Carmen Fault to the south. The mid-ocean ridge system is responsible for the creation of the Guaymas Basin and giving it many features such as hydrothermal circulation and hydrocarbon seeps. Hydrothermal circulation is a significant process in the Guaymas Basin because it recycles energy and nutrients which are instrumental in sustaining the basin's rich ecosystem. Additionally, hydrocarbons and other organic matter are needed to feed a variety of organisms, many of which have adapted to tolerate the basin's high temperatures.

The oceanic carbon cycle is composed of processes that exchange carbon between various pools within the ocean as well as between the atmosphere, Earth interior, and the seafloor. The carbon cycle is a result of many interacting forces across multiple time and space scales that circulates carbon around the planet, ensuring that carbon is available globally. The Oceanic carbon cycle is a central process to the global carbon cycle and contains both inorganic carbon and organic carbon. Part of the marine carbon cycle transforms carbon between non-living and living matter.

Marine biogeochemical cycles are biogeochemical cycles that occur within marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. These biogeochemical cycles are the pathways chemical substances and elements move through within the marine environment. In addition, substances and elements can be imported into or exported from the marine environment. These imports and exports can occur as exchanges with the atmosphere above, the ocean floor below, or as runoff from the land.

The copper cycle is the biogeochemical cycle of natural and anthropogenic exchanges of copper between reservoirs in the hydrosphere, atmosphere, biosphere, and lithosphere. Human mining and extraction activities have exerted large influence on the copper cycle.

The boron cycle is the biogeochemical cycle of boron through the atmosphere, lithosphere, biosphere, and hydrosphere.

The arsenic (As) cycle is the biogeochemical cycle of natural and anthropogenic exchanges of arsenic terms through the atmosphere, lithosphere, pedosphere, hydrosphere, and biosphere. Although arsenic is naturally abundant in the Earth's crust, long-term exposure and high concentrations of arsenic can be detrimental to human health.

The lead cycle is the biogeochemical cycle of lead through the atmosphere, lithosphere, biosphere, and hydrosphere, which has been influenced by anthropogenic activities.

The fluorine cycle is the series of biogeochemical processes through which fluorine moves through the lithosphere, hydrosphere, atmosphere, and biosphere. Fluorine originates from the Earth’s crust, and its cycling between various sources and sinks is modulated by a variety of natural and anthropogenic processes.

The prebiotic atmosphere is the second atmosphere present on Earth before today's biotic, oxygen-rich third atmosphere, and after the first atmosphere of Earth's formation. The formation of the Earth, roughly 4.5 billion years ago, involved multiple collisions and coalescence of planetary embryos. This was followed by a <100 million year period on Earth where a magma ocean was present, the atmosphere was mainly steam, and surface temperatures reached up to 8,000 K (14,000 °F). Earth's surface then cooled and the atmosphere stabilized, establishing the prebiotic atmosphere. The environmental conditions during this time period were quite different from today: the Sun was ~30% dimmer overall yet brighter at ultraviolet and x-ray wavelengths, there was a liquid ocean, it is unknown if there were continents but oceanic islands were likely, Earth's interior chemistry was different, and there was a larger flux of impactors hitting Earth's surface.

The manganese cycle is the biogeochemical cycle of manganese through the atmosphere, hydrosphere, biosphere and lithosphere. There are bacteria that oxidise manganese to insoluble oxides, and others that reduce it to Mn2+ in order to use it.














